Alzheimer Disease
Definition
Alzheimer disease (AD) is histopathologically defined by neurofibrillary tangles and neuritic plaques in the cerebral cortex. AD is also commonly used as a clinical diagnosis for a dementia syndrome in which anterograde amnesia is a dominant symptom. The most common pathologic basis for the clinical syndrome AD is indeed the pathologically defined disease AD.
A person with the dementia of AD experiences a loss of cognitive function that interferes with social or occupational activities and generally results in a dependence on others for carrying out at least some necessary daily affairs. In the language of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition: "The cognitive deficits cause significant impairment in social and occupational functioning and represent a significant decline from a previous level of functioning."1 The decline from a previously higher level of functioning distinguishes dementia from developmental delays or mental retardation. The sensorium (i.e., the person’s level of arousal and alertness) is unaffected in dementia. This feature distinguishes dementia from delirium, in which cognition is impaired in the setting of an altered sensorium.
As defined, dementia is impairment of more than one area of cognitive function. In the dementia syndrome of AD, therefore, anterograde amnesia must be accompanied by impairment of at least one of the following: language, abstract reasoning, executive functioning, and visuospatial processing. The most widely used definition of AD is that based on criteria established by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer Disease and Related Disorders Association (NINCDS-ADRDA) work group2 [see Table 1].
The pivotal cognitive finding in AD is anterograde amnesia, which is an inability to learn new things. Persons with antero-grade amnesia typically cannot keep track of the date, remember recent conversations, or remember where they set something down; and they often repeat themselves in conversation. Although these types of difficulties are commonly described as short-term memory loss, the key difficulty in anterograde amnesia of AD is in acquiring or learning new information. Failure to learn results in difficulties recalling related information a few minutes later. AD is not a generalized disorder of recall, however, because early in the disease—especially, but even into its moderate stages—persons with AD can retrieve and recall learned information from the remote past.
Epidemiology
The prevalence of AD is approximately 8% of the United States population older than 65 years.3 Approximately two to three million persons in the United States have AD; estimates vary somewhat because of different case definitions used.3 If very mild AD cases are included, the number of affected persons exceeds four million.4 The prevalence is strongly age related [see Figure 1]: AD is uncommon in persons younger than 65 years, but prevalence doubles every 5 years from then on. As many as 30% to 40% of persons older than 85 years have AD.
The incidence of AD mirrors the prevalence, being very low before 65 years of age and then steadily increasing into the 70s and 80s. By the age of 80, the annual incidence of AD approaches two per 100 persons. There is some controversy regarding the incidence of AD in the 10th and 11th decades of life; it probably continues to rise, but the methodology and numbers of cases at the extremes of life expectancy make for uncertainty.
There is also controversy as to whether women are more commonly affected than men. Most studies of prevalence and incidence show that women are slightly overrepresented. The difference is small, however, and could be an artifact of the overall lower life expectancy of men.5 Emerging studies of dementia in African Americans6 and Japanese Americans7 suggest that there are no ethnic differences in the prevalence or incidence of AD.
Protective and risk factors
Several factors appear to alter the risk of developing AD. The most prominent risk factors for AD are advancing age and a family history of dementia. Cardiovascular disease8 and diabetes mellitus9 both appear to be associated with an increased risk of AD. Elevated levels of homocysteine also are associated with a higher risk of AD.
|
Table 1 Criteria for the Clinical Diagnosis of Alzheimer Disease* |
|
A. Evidence from the history and mental-status examination that indicates a disorder characterized by major impairment of learning and of retaining new information, as well as at least one of the following: |
|
1. Impairment in handling complex tasks |
|
2. Impairment in reasoning ability |
|
3. Impaired spatial ability and orientation |
|
4. Impaired language |
|
B. The disturbances listed under A above significantly interfere with work or usual social activities or relationships with other people. |
|
C. The disturbances represent a significant decline from a previous level of functioning. |
|
D. The disturbances are of insidious onset and are progressive, based on evidence from the history or serial mental-status examinations. |
|
E. The disturbances are not occurring exclusively during the course of delirium. |
|
F. The disturbances are not better accounted for by a major psychiatric diagnosis. |
|
G. The disturbances are not better accounted for by a systemic disease or another brain disease. |
"These criteria are based on the National Institute of Neurological and Communicative Disorders and Stroke and the
Alzheimer Disease and Related Disorders Association work group report2 and the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.1
Figure 1 Prevalence of Alzheimer disease per 1,000 population, by gender and age.
Use of certain medications is associated with a lower risk of developing AD. These include estrogen, the statin-type cholesterol-lowering drugs, and nonsteroidal anti-inflammatory drugs (NSAIDs). For estrogen and the NSAIDs, medication use was assessed when persons were not demented, a methodological strategy that strengthened the belief that the observed reduction in risk was not an artifact of the appearance of early AD. Unfortunately, subsequent clinical trials of estrogen11 and NSAIDs12 in AD patients, as well as a very large prevention study using estrogen and progesterone,13 have uniformly failed to show evidence of benefits for either class of drug. On the basis of the same types of observations,14 clinical trials with statins in AD are under way. The disappointing study conclusion with estrogen and NSAIDs is that epidemiologic associations do not automatically translate into successful therapeutic interventions.
Level of educational attainment and intellectual performance in childhood also appear to be related to the risk of developing AD in later life. In a study of persons who took academic achievement tests at 11 years of age and then were evaluated in adulthood for dementia, there was a notable association between higher scores at age 11 and a lower likelihood of developing AD in later life.15 Similarly, a study of elderly Catholic nuns who had written autobiographies when they entered the order, at approximately age 20, showed that the nuns with better vocabularies and syntactic abilities when young had a lower likelihood of developing AD in later life.16 Many other studies have observed that educational attainment seems to be inversely related to the risk of AD, though questions have been raised as to whether the so-called education effect is actually of socioeconomic origin.
Genetics and molecular biology
The genetic basis of AD can be divided into four profiles: (1) the very rare autosomal dominant forms of AD; (2) the more common, later-onset form of AD, in which family history exerts a modestly strong risk; (3) sporadic AD, which comprises the majority of AD patients, including both early-onset and, especially, late-onset forms that appear to be nongenetic; and (4) persons with Down syndrome (trisomy 21).
The number of persons with autosomal dominant AD is infinitesimal compared with the large number of persons with late-onset sporadic AD. The autosomal dominant families have been pivotal, however, in providing insights into the mechanisms of the disease. In general, affected individuals in autosomal dominant AD families experience dementia in their 30s to 60s; the genetic disorder advances the age of onset of AD by roughly 2 to 3 decades relative to sporadic AD.
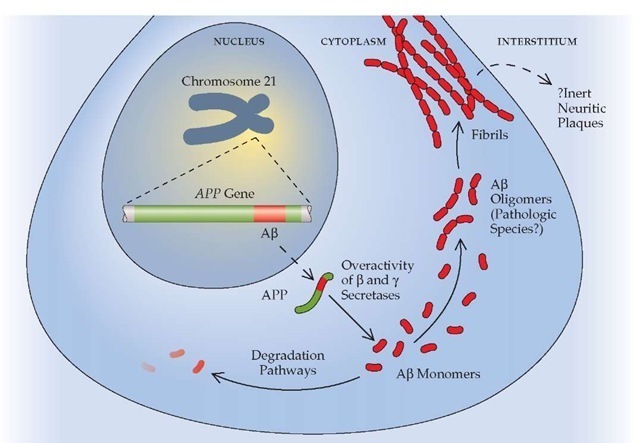
Three genes have been implicated in autosomal dominant AD. One is the amyloid precursor protein (APP) gene, on chromosome 21, which encodes the amyloid-P peptide [see Figure 2]. The amyloid-P peptide appears to be a key pathogenic molecule. Only a handful of families in the world harbor the mutations in this gene that cause AD. The other two genes involved in autoso-mal dominant AD are homologous; the much more common of the two is the presenilin-1 (PS-1) gene, located on chromosome 14. Approximately half of the few hundred autosomal dominant AD families have disease linked to mutations on chromosome 14.18 The less common homologue, presenilin-2 (PS-2), is encoded by a gene on chromosome 1.19 Families with the PS-2 mutation are almost exclusively of one ethnic group—Germans whose ancestors emigrated to the western United States and Canada after a sojourn of a few hundred years in the Volga River area of Russia. The mutation is thought to have resulted from a founder effect, which propagated by virtue of the cultural isolation in a foreign land of this group of people.
In addition to the rare autosomal dominant families, a family history of AD, even of later onset, is a consistent risk factor for the disease.20 One early study showed that the risk of AD in siblings of probands with autopsy-proven AD rose to nearly 50% by age 85 if two features were present: the presence of dementia in another first-degree relative and an age of onset under 70 years in the proband.21 Subsequently, as families with late-onset AD were studied genetically, the apolipoprotein E (APOE) gene was found to account for a substantial fraction of the familial risk in late-onset AD.22,23 The APOE gene has three allelic variations (e2, e3, and e4) in humans, as a result of polymorphisms in two nucleotides. The APOE e4 allele is strongly associated with the development of AD at an earlier age than in non-e4 carriers.24 However, the presence of an APOE e4 allele does not guarantee the development of AD in APOE e4 carriers, and its absence does not protect against AD in APOE non-e4 carriers.25 There are no clinical differences between AD patients with and AD patients without the APOE e4 allele. No other gene has been linked definitively to risk of AD. Although there is intense interest in a region on chromosome 10, the exact gene has not been identified.
Progress in defining the molecular biology of AD has provided the framework for understanding the probable pathophysio-logic pathways that lead to AD [see Figure 2]. In essence, the so-called amyloid hypothesis of AD asserts that the amyloid-P pep-tide is toxic either to neurons or to synaptic function in a dose-dependent fashion.26,27 According to this theory, excess production or reduced clearance of the amyloid-P peptide will lead to AD, and the higher the amyloid-P peptide load, the earlier the onset of the dementia. Individuals with Down syndrome also exhibit AD pathology 3 to 4 decades earlier than that which is seen in patients with sporadic AD, presumably because of the extra chromosome 21 that contains the wild-type APP gene. The APP gene mutations lead to overproduction of the amyloid-P peptide because the mutations upset the balance of the three se-cretases involved in APP metabolism. The PS-1 and PS-2 genes appear to play an integral role in the action of one of those secre-tases, the y-secretase. The mutations in PS-1 and PS-2 result in a heightened activity of y-secretase that also leads to excess production of the amyloid-3 peptide. Individuals with the APOE e4 allele also have excess amyloid-3 peptide in their brains, but the precise mechanism by which the APOE genotype modifies the molecular biology of AD is still a matter of debate.
Figure 2 Schematic overview of the amyloid-3 peptide production pathway. The Alzheimer precursor protein (APP) is encoded by a gene on chromosome 21. Under certain circumstances, presumably involving relative overactivity of 3 and y secretases compared with a secretase, the amyloid-3 peptide is produced. It may assemble itself into dimers, trimers, or other oligomers, which are thought to be cytotoxic. Eventually, the oligomers polymerize into fibrils and, later, into plaques.
Pathophysiology
The brain of a patient who has died with the dementia syndrome of AD will usually show gross atrophy of the cerebral hemispheres, particularly in the posterior half of the brain. Microscopic examination of the brain is required to make a definitive diagnosis of AD, however.
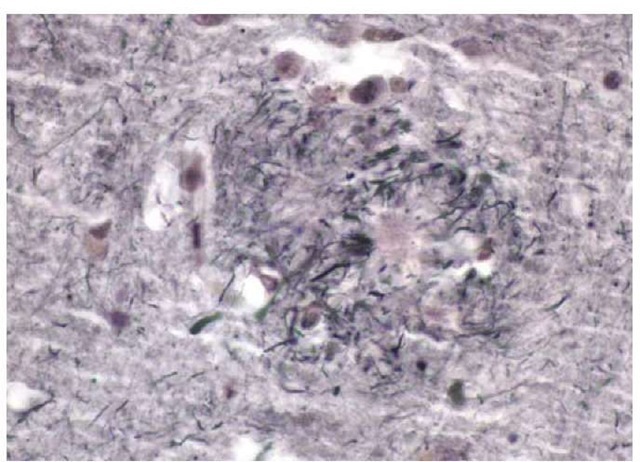
There are two principal histologic findings in AD. The first is the neuritic plaque. Neuritic plaques are extracellular structures that contain a core made up of the amyloid-3 peptide and surrounded by degenerated axonal and dendritic elements of many neurons [see Figure 3]. They can be imaged with routine hema-toxylin and eosin staining, but they are best seen with silver stains. Neuritic plaques may be present in very small numbers in nondemented elderly persons, but in the typical patient with AD, neuritic plaques are numerous28 and are located in many parts of the cerebral cortex.29
The genesis of neuritic plaques is better understood than it once was, but there are still gaps in the described pathway between an excess of the amyloid-3 peptide and the appearance of neuritic plaques. It is currently believed that the amyloid-3 pep-tide polymerizes and that the oligomer consisting of a few copies of the amyloid-3 peptide is neurotoxic. Presumably, the amy-loid-3 peptide oligomers continue to aggregate and eventually form the amyloid core of the neuritic plaques. Many experts believe that the oligomeric form of the amyloid-3 peptide is the key pathogen in AD30 and that the neuritic plaques merely represent the end stage of the pathologic process.
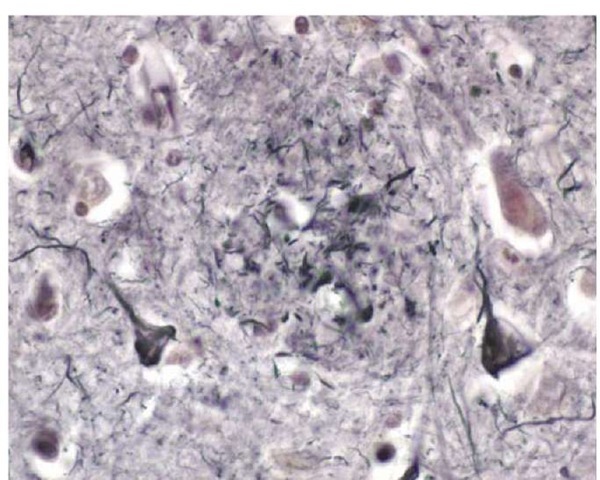
The other principal histologic feature in AD is the intracel-lular neurofibrillary tangle [see Figure 4]. Neurofibrillary tangles are found inside neurons and consist of a protein known as tau that has been pathologically modified so that it has polymerized into an insoluble form that assembles itself into filaments. Neurofibrillary tangles may be seen in nondement-ed elderly persons in the entorhinal cortex and even in the hippocampus, but when neurofibrillary tangles are found in larg-er numbers in the neocortex of the temporal or parietal lobes, the person will almost invariably have had AD dementia.
Figure 3 Photomicrograph of a neuritic plaque in the cortex of a patient with Alzheimer disease.
Figure 4 Photomicrograph of several neurons containing neurofibrillary tangles from the cortex of a patient with Alzheimer disease.
The tau protein is the most important molecule in neurofibril-lary tangles. It is ordinarily involved in stabilizing the cytoskele-tal elements in neurons (i.e., the microtubules). In AD, tau becomes excessively phosphorylated and aggregated.32 Curiously, despite the discovery of mutations in the tau gene that cause other forms of dementia, no tau gene mutations have been associated with AD. It seems most likely that formation of neurofibril-lary tangles represents a technically normal but ultimately disastrous response to injury caused by the amyloid-P peptide. The amyloid-P peptide induces excessive phosphorylation of tau protein, which destabilizes microtubules and leads to aggregation of hyperphosphylated tau into fibril.