Biomedical Engineering Reference
In-Depth Information
↔
N
-Hcy-SH-transferrin + albumin-Cys
34
-S-S-Cys
↔
N
-(Hcy-S-S-Cys)-transferrin + albumin-Cys
34
-SH
Reaction 5.5 Thiol-disulfide exchange between N-Hcy-transferrin and albumin-Cys
34
-S-S-Cys.
Similar reactions occur with other N-Hcy-proteins, such as N-Hcy-fibrinogen, N-Hcy-antitrypsin,
N-Hcy-hemoglobin, N-Hcy-myoglobin, and N-Hcy-cytochrome c
Hcy-SH
S-S-Cys
S-S-Cys
H
2
N
HN
Lys
525
Cys
34
+ Hc
y-thiolac
tone
Cys
34
Lys
525
Hcy-S-S-Cys
HN
SH
Lys
525
Cys
34
Hcy-SH
H
2
N
SH
HN
SH
+ H
cy-thiolac
tone
Lys
525
Cys
34
Lys
525
Cys
34
Fig. 5.9 N-Homocysteinylation of Lys
525
prevents the structural transition in albumin dependent
on the status of the conserved Cys
34
residue (Reproduced from [69])
Of the seven lysine residues of human albumin (Lys
4
,Lys
12
,Lys
137
,Lys
159
,Lys
205
,
Lys
212
,andLys
525
) identified as targets for N-homocysteinylation by Hcy-thiolactone
in vitro, Lys
525
is a predominant site of N-homocysteinylation, while Lys
137
and Lys
212
are minor sites, both in vitro and in vivo (Fig.
5.2
) [212, 213]. Taken together, these
results provide evidence for a novel form of albumin, N-(Hcy-S-S-Cys)-albumin-
Cys
34
-SH (Figs.
5.7
and
5.9
), and suggest that a disulfide at Cys
34
, a conserved residue
in albumins from various organisms, promotes the conversion of N-(Hcy-SH)-albumin-
Cys
34
-SH to a more proteolytically sensitive form N-(Hcy-S-S-Cys)-albumin-Cys
34
-
SH, which would facilitate clearance of the N-homocysteinylated form of
mercaptoalbumin. These data also suggest that, by rendering Cys
34
reduced, N-
homocysteinylation prevents the structural transition in albumin dependent on the
status of the conserved Cys
34
residue (Fig.
5.9
).
N-Hcy-albumin becomes more susceptible than native albumin to aggregation
[78] and oxidative damage by hydrogen peroxide [96]. Exposure to N-Hcy-albumin
increases monocyte adhesion to the endothelial monolayers in a co-culture model
[169]. Unmodified albumin does not have any effect on monocyte adhesion. The
increased adhesion is observed only when N-Hcy-albumin is used at concentration
observed in hyperhomocysteinemic subjects, but not when the concentration
corresponds to that observed in normal individuals. The increased cell adhesion is
accompanied by upregulation of genes involved in the inflammatory response
(ICAM-1, VCAM-1) and vascular remodeling (ADAM17, MCP1, Hsp60) in both
endothelial cells and monocytes. N-Hcy-albumin also induces release of Tnf-
from
endothelial cells to the medium. As these responses are observed at relatively low
concentration of N-Hcy-albumin, these findings suggest that N-Hcy-protein rather
α






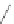
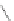
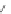






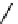
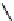

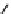


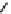

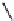

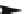







Search WWH ::

Custom Search