Biomedical Engineering Reference
In-Depth Information
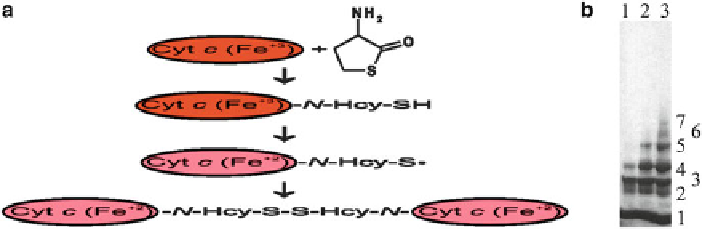
Fig. 5.10 N-Homocysteinylation of cytochrome c renders it reduced and leads to oligomerization.
Left panel: Schematic illustration of the mechanism of the modification of cytochrome c with Hcy-
thiolactone. Oxidized and reduced forms of cytochrome c are shown as red and green ovals,
respectively. Right panel: SDS-PAGE analysis of N-Hcy-cytochrome c oligomers on 4-20 % gels.
Bovine cytochrome c (10 mg/mL) was modified for 24 h at 25
C with 30 μM (lane 1), 600 μM
(lane 2), and 2.5 mM (lane 3) [
35
S]Hcy thiolactone. The samples were denatured in the absence of
2-mercaptoethanol and subjected to SDS-PAGE. An autoradiogram of the gel is shown. The
patterns of
35
S-labeled bands are identical to the patterns of red cytochrome c bands (not shown).
Unmodified cytochrome c migrates as a single band (not shown). Numbers from 1 to 7 next to the
bands indicate cytochrome c monomers, dimers, trimers, etc., respectively, as determined by
comparison with migration of protein molecular mass standards (Reproduced from [78] and [298])
than Hcy itself induces cellular responses leading to chronic upregulation of
inflammatory chemokines/cytokines, which are involved in atherosclerotic lesion
formation [169].
5.4.1.2
N
-Hcy-Cytochrome
c
Studies of the modification of cytochrome c by Hcy-thiolactone provide a paradigm
illustrating how the function of a heme-containing protein can be affected by N-
homocysteinylation [298]. Four lysine residues of cytochrome c, Lys8 or 13, Lys86
or 87, Lys99 and Lys100, are preferential sites for the modification by Hcy-
thiolactone in vitro. N-homocysteinylation of ferricytochrome c results in its con-
version to a ferrous form, which is manifested as a change in the color of the
solution from red to green. Experimental data are consistent with the following
mechanism (Fig.
5.10
). Reaction of Hcy-thiolactone with any of the four suscepti-
ble lysine residues of ferricytochrome c affords N-(Hcy-SH)-Cyt c(Fe
+3
). The heme
iron in the product undergoes reduction by the thiolate of N-linked Hcy to afford
modified ferrocytochrome c, N-(Hcy-S
)-Cyt c(Fe
+2
). The reduction occurs in trans
between different molecules of N-(Hcy-SH)-Cyt c(Fe
+3
) and can also occur with
other N-Hcy-proteins. For example, a similar reduction of heme-Fe
+3
was also
observed during incubation of ferricytochrome c with N-(Hcy-SH)-albumin [298].
An intramolecular reduction is unlikely, because the sites of N-homocysteinylation
are located too far from the heme iron. Dimerization of the thiyl radicals in different
molecules of the modified ferrocytochrome c, N-(Hcy-S
∙
)-Cyt c(Fe
+2
), leads to the
∙
Search WWH ::

Custom Search