Biomedical Engineering Reference
In-Depth Information
a
b
c
Alb-Cys-S-S-Cys
250
30
N
-(Hcy-S-S-Cys)-Alb-Cys-SH
Alb-Cys-S-S-Cys
20
200
N
-(Hcy-S-S-Cys)-Alb-Cys-SH
10
150
Alb-Cys-S-S-Cys
N-
(Hcy-S-S-Cys)
-
Alb-Cys-S-S-Cys
0
10
100
N-
(Hcy-S-S-Cys)-
N
-([
35
S]Hcy-S-S-Cys)-Alb-Cys-SH
3,000
A
lb-Cys-SH
N
-(Hcy-S-S-Cys)- Alb-Cys-SH
50
2,000
5
1,000
0
0
0
12
13
14
12
13
14
15
11
12
13
14
15
Minutes
Minutes
Minutes
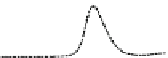
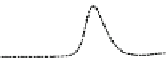
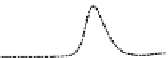
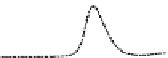
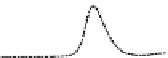
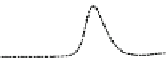
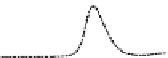
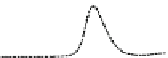
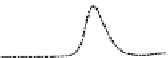
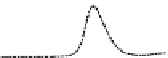
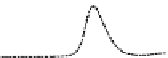
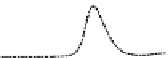
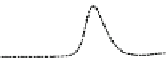
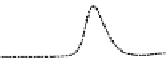
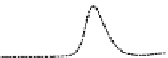
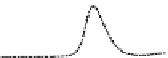
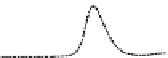
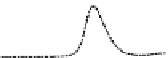
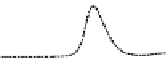
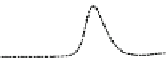
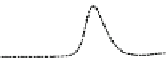
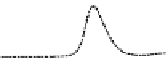
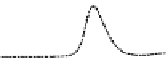
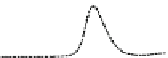
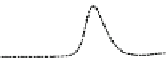
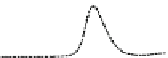
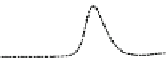
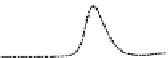
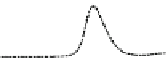
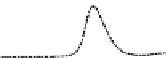
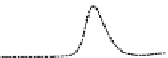
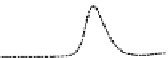
Fig. 5.8 Anion exchange HPLC analysis of Hcy-thiolactone-modified albumin-Cys
34
-S-S-Cys.
Albumin-Cys
34
-S-S-Cys was modified with Hcy-thiolactone or [
35
S]Hcy-thiolactone at 37
C and
analyzed by anion exchange HPLC. Panel (a), protein profiles after 0-h (top trace), 4-h (middle
trace), and 22-h (bottom trace) modification with Hcy-thiolactone. Panel (b) shows protein profiles
of the 10-h reaction with Hcy-thiolactone after an overnight incubation without (dotted line) and
with a twofold molar excess of cysteine (solid line). Panel (c), protein (upper panel) and
35
S(lower
panel) profiles after 4 h of modification with [
35
S]Hcy-thiolactone (Reproduced from [96])
Hcy-thiolactone + albumin-Cys
34
-S-S-Cys
→
→
N
-(Hcy-SH)-albumin-Cys
34
-S-S-Cys
↔
N
-(Hcy-S-S-Cys)-albumin-Cys
34
-SH
Reaction 5.3 Mechanism of N-homocysteinylation of albumin-Cys
34
-S-S-Cys
→
N
-(Hcy-SH)-albumin-Cys
34
-SH
(a)
Hcy-thiolactone + albumin-Cys
34
-
-SH
N
-Hcy-SH-albumin-Cys
34
-SH + albumin-Cys
34
-S-S-Cys
↔
↔
N
-(Hcy-S-S-Cys)-albumin-Cys
34
-SH + albumin-Cys
34
-SH
(b)
Reaction 5.4 Mechanism of N-homocysteinylation of mercaptoalbumin (albumin-Cys
34
-SH)
The formation of a mixed disulfide bond between Cys and N-linked Hcy induces
substantial structural changes that lead to increased sensitivity of N-(Hcy-S-S-Cys)-
albumin-Cys
34
-SH, compared with N-(Hcy-SH)-albumin-Cys
34
-SH, to proteolysis
by trypsin or chymotrypsin [96].
Other plasma N-Hcy-proteins also undergo facile thiol-disulfide exchange with
albumin-Cys
34
-S-S-Cys. For example, when equimolar amounts of N-Hcy-trans-
ferrin and albumin-Cys
34
-S-S-Cys are incubated together, albumin-Cys
34
-S-S-Cys
is quantitatively converted to albumin-Cys
34
-SH (Reaction
5.5
). Unmodified trans-
ferrin does not induce this reaction. N-homocysteinylated fibrinogen, antitrypsin,
hemoglobin, myoglobin, and cytochrome c, but not unmodified native proteins, also
removed cysteine from the albumin-Cys
34
-S-S-Cys disulfide with the liberation of
albumin-Cys
34
-SH. The equilibrium of those reactions is strongly shifted toward
N-(Hcy-S-S-Cys)-protein and albumin-Cys
34
-SH [96].

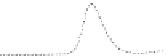

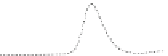

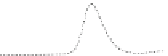

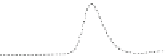

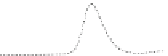

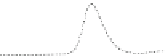

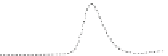

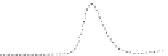
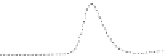

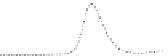

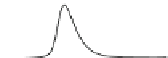
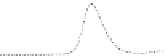


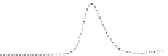

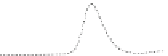

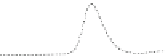

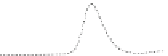
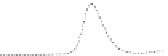

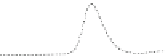

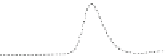

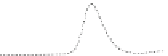

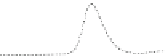

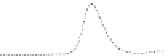

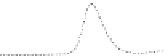

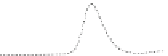
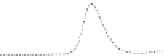

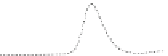

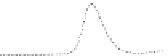
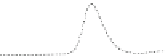

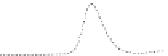
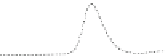

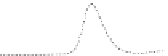

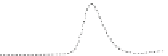

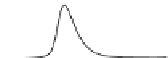
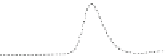


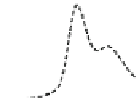


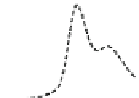


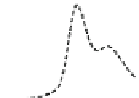



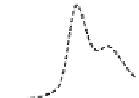


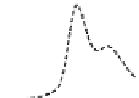


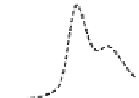


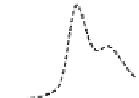



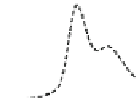


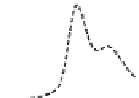


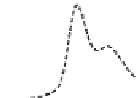


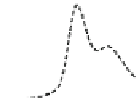



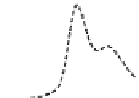

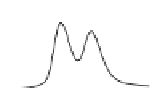


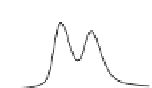


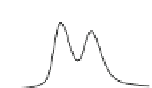








































































































































Search WWH ::

Custom Search