Biomedical Engineering Reference
In-Depth Information
SH
ALBUMIN
Albumin-Cys
34
-SH
S-S-Cys
ALBUMIN
Albumin-Cys
34
-S-S-Cys
S-S-Hcy
ALBUMIN
Albumin-Cys
34
-S-S-Hcy
NH
2
H
N
SH
ALBUMIN
HS
C
O
N
-(Hcy-SH)-Albumin-Cys
34
-SH
NH
2
H
N
SH
ALBUMIN
Cys-S-S
C
O
N
-(Hcy-S-S-Cys)-Albumin-Cys
34
-SH
NH
2
H
N
S-S-Cys
ALBUMIN
Cys-S-S
C
O
N
-(Hcy-S-S-Cys)-Albumin-Cys
34
-S-S-Cys
Fig. 5.7 Structures of the different forms of human serum albumin. Reproduced from [96]
albumin is chromatographically separated from unmodified albumin by anion
exchange HPLC (Fig.
5.8
). The different susceptibilities of albumin-Cys
34
-
S-S-Cys and albumin-Cys
34
-SH to the modification by Hcy-thiolactone are
consistent with a structural transition in albumin dependent on the status of the
Cys
34
residue [324].
The reactions of albumin-Cys
34
-S-S-Cys and albumin-Cys
34
-SH with
Hcy-thiolactone yield two different primary products, N-(Hcy-SH)-albumin-
Cys
34
-S-S-Cys (Reaction
5.3
) and N-(Hcy-SH)-albumin-Cys
34
-SH (Reaction
5.4
),
respectively (Fig.
5.9
). However, these primary products are not observed due to
fast thiol-disulfide exchange reactions that result in the formation of a single
product, N-(Hcy-S-S-Cys)-albumin-Cys
34
-SH (Fig.
5.9
), which is observed on an
anion exchange column (Fig.
5.8
).
The thiol-disulfide exchange reactions occur in trans between different
molecules of N-(Hcy-SH)-albumin-Cys
34
-S-S-Cys or between N-(Hcy-SH)-
albumin-Cys
34
-SH and albumin-Cys
34
-S-S-Cys. The equilibrium is strongly shifted
toward N-(Hcy-S-S-Cys)-albumin-Cys
34
-SH because the Cys-34 thiolate anion has
unusually low pK
a
of ~5 [323] and thus is more thermodynamically stable that Hcy
thiolate anion (pK
a
of ~10) [191, 192]. The low pK
a
of the Cys-34 thiolate also
makes the thiol-disulfide exchange of N-(Hcy-SH)-albumin-Cys
34
-SH with
albumin-Cys
34
-S-S-Cys thermodynamically more favored than with cystine.


















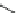







Search WWH ::

Custom Search