Chemistry Reference
In-Depth Information
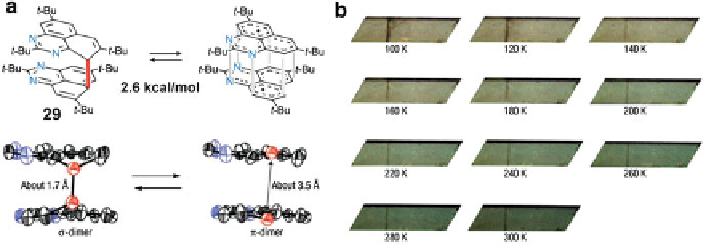
Fig. 7 (a) Chemical structure and X-ray single-crystal structure of
s
-dimer and
p
-dimer for 29;
(b) images of crystal of 29 in the range of temperature between 100 K and 300 K [
22
]. Reprinted
with permission from [
22
]. Copyright 2008 Nature Publishing Group
to the thermally accessible triplet state. This is the first example where the energy
levels of an organic radical with three energy states have been experimentally
located. On the other hand, radical 10 did not show any thermochromism in the
crystalline phase, suggesting that the symmetry lowering of the spin distribution
plays a pivotal role to form a
-dimer equilibrium [
22
].
A highly symmetrical nitrogen-containing phenalenyl 22 with all
a
positions
replaced by nitrogen atoms is a very interesting motif in that the nitrogen atoms are
directed radially and can form coordination and hydrogen bonds to the outside. Due
to the largely decreased HOMO energy level, the anion of this compound possesses
high stability and can be isolated as stable crystals at room temperature in air [
23
].
Two
s
-dimer and
p
type azaphenalenyls 23 and 30 were prepared by Rubin et al. For 23, the
substitution of carbon with nitrogen increases the stability in degassed solution, but
the low stability in air and in the solid state hampered its isolation in the crystalline
form [
24
]. On the other hand, radical 30 exhibited high stability both in the solid
state and in air, but a dechlorinated dimer connected with a C
b
C bond was found to
form. The ESR measurements and density functional theory (DFT) calculations of
those radicals indicated large spin densities on the
¼
a
positions, which is similar to
the pristine phenalenyl radical [
25
] (Fig.
8
).
The above-mentioned systems possess similar electronic structures with
phenalenyl radicals, having large positive spin densities on the
positions. In
contrast, introduction of two oxygen atoms will lead to a brand new series of
radicals termed “oxophenalenoxyls” [
26
]. These neutral radicals are different
from phenalenyl radicals in the following aspects: (1) the
a
-substituted isomer
can be represented as triradical structure and is much less stable than the
b
,
b
a
,
b
a
a
-substituted isomers which can be represented as monoradicals;
(2) high stability can be expected for the
-substituted or
,
a
a
-substituted isomer due to the extensive
delocalization of radicals and unique topological symmetries; (3) most of the spin
densities and coefficients of SOMO are centered on the
b
carbons and the oxygen
atoms, and significant SOMO level lowering compared to phenalenyl systems
imply a high electron accepting ability, which is the key factor for applications as
electrode active materials in secondary batteries.
,