Chemistry Reference
In-Depth Information
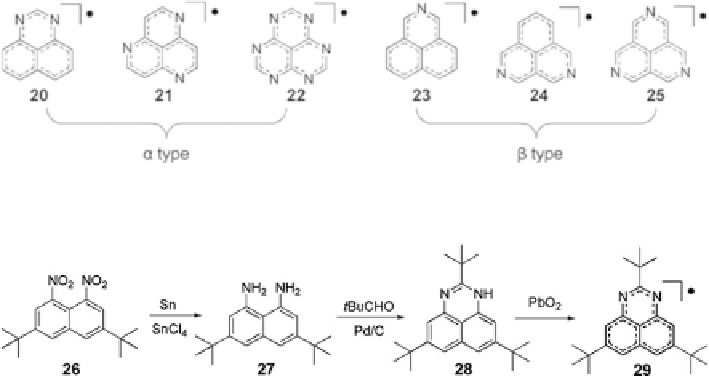
Fig. 6 Nitrogen-containing phenalenyl radicals [
1
]
Scheme 3 Synthetic route to the radical 29 [
21
]
density distributions are similar to the pristine phenalenyl radicals, with a slight
decrease of spin densities on nitrogen atoms. In contrast,
type compounds show
minimal perturbation on the electronic structure as the nitrogen atoms locate at
positions with small and negative spin densities.
The first synthesis and isolation of an
b
type nitrogen-containing phenalenyl
radical, a 2,5,8-tri-
tert
-butyl-1,3-diazaphenalenyl 29, was achieved in 2002. The
synthesis involved reduction of compound 26 followed by condensation with
t
BuCHO and dehydrogenation catalyzed by Pd/C. Oxidation with PbO
2
and recrys-
tallization led to a green crystal of 29. The crystal exhibited increased stability in air
due to heteroatomic modification [
21
] (Scheme
3
).
Due to the symmetry-broken incorporation of two nitrogen atoms at the two
a
a
positions, the X-ray analysis of single crystals of 29 revealed formation of an angled
face-to-face
syn
-dimer structure and a strongly asymmetric bonding nature in the
crystal that showed large temperature factors and high anisotropy at 296 K (Fig.
7
).
These features were associated with a continuous color change from 100 K to
300 K. The crystal was colorless or an extremely pale green at 100 K, which
gradually deepened at higher temperature and finally became dark green at
300 K. This behavior is very rare not only in organic radicals but also in metal
complexes, making the crystal of 29 a molecular color thermometer. With the help
of X-ray single-crystal analysis and temperature dependant polarized electronic
spectra, this unconventional dynamic behavior was found to originate from the
coexistence of
-bonded dimers, separated by an energy gap of
2.62 kcal mol
1
. The temperature dependent polarized electronic spectra showed
that the
s
-bonded and
p
-dimer were colorless and dark green, respectively, so the
continuous color change originated from the change of population of the two dimers
with respect to temperature. The presence of two dimers was further supported by
room temperature ESR measurement of the solid, which showed signals attributed
s
-dimer and
p