Chemistry Reference
In-Depth Information
Br
HO
O
COOCH
3
1.
n
BuLi, DMF
1. Et
3
SiH, TFA
2.
t
BuCHBrCO
2
CH
3
Zn
2. Lil, DMF
3. (COCl)
2
4.
AlCl
3
6
7
8
C20
C22
C21
C15
1. LiAlH
4
p
-chloranil
C8
C7
C9
C6
C10
2.
p
-TsOH
Ct
C5
C11
C17
C14
C18
C4
C12
C25
C16
9
C2
10
C13
C23
C3
C19
C24
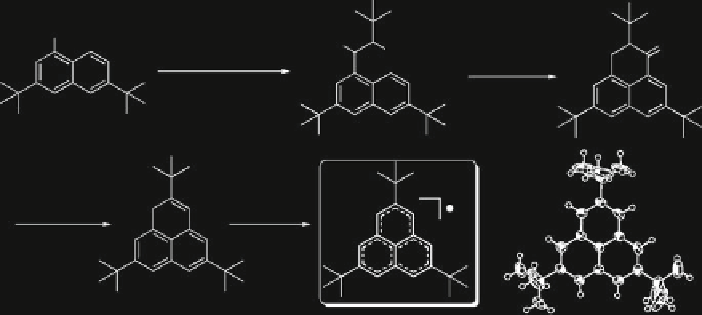
Scheme 1 Synthetic route and X-ray crystal structure of radical 10 [
7
]
displayed enhanced stability in solution. The first isolation of phenalenyl in the
solid state in air was achieved in 1999 when Nakasuji et al
.
introduced three
tert
-
butyl groups onto the
b
positions. The bulky substituents not only successfully shut
down the
-bond dimerization pathway but also contributed a minimal perturbation
effect to the electronic structure of the parent phenalenyl in that the connection
positions were
s
positions with negligible spin densities. Therefore, it can be
viewed as the most electronically fundamental phenalenyl-based system [
7
].
As shown in Scheme
1
, the synthesis of
tert
-butyl phenalenyl radical 10 started
from 2,7-di-
tert
-butylnaphthalene in ten steps. Bromination of 2,7-di-
tert
-
butylnaphthalene gave 6 in high yield, which was converted into aldehyde by
lithiation followed by reaction with DMF. Successive Reformatsky reaction
afforded ester 7, which upon reduction, hydrolysis, and Friedel-Crafts acylation
reaction gave the phenalanone 8. The key intermediate 9 was then obtained as pale
yellow crystals by reduction of 8 and subsequent dehydration. Oxidation of 9 with
p
-chloranil in degassed toluene led to a blue neutral radical solution while similar
treatment in hexane gave deep blue needles. This crystal showed high stability in
the absence of air, while changing into phenalanone derivatives and other
byproducts in 1 week in air.
The ESR spectrum of 10 showed a septet hyperfine structure corresponding to
phenalenyl ring protons, and an observed
g
e
value 2.0028, consistent with genuine
spin-doublet hydrocarbon radicals. The X-ray analysis of 10 was reported for the first
time for an odd alternant hydrocarbon radical, revealing that it possesses a nearly
planar geometry with a slightly distorted
D
3h
symmetry. The molecule formed a
p
b
-dimeric pair in staggered alignment of the
tert
-butyl groups to avoid steric repul-
sion, which also favored the maximum SOMOs overlap. The dimeric pair adopted a
herringbone packing motif with interplanar distance ranging from 3.201 to 3.323
´
,
much shorter than the sum of the van der Waals radii of the carbon atoms. Such a
short distance indicated a strong antiferromagnetic interaction within the
-dimer,
which was evidenced by a large antiferromagnetic intermolecular exchange
p