Chemistry Reference
In-Depth Information
a
t
-Bu
b
1.48
*
143
128
123
120
t
-Bu
t
-Bu
Top view
Side view
*
10
6.47
*
*
180 K
185 K
190 K
200 K
210 K
230 K
250 K
270 K
*
180 K
*
t
-Bu
*
*
*
*
t
-Bu
t
-Bu
t
-Bu
t
-Bu
270 K
140 120
13
C NMR
ppm
7
6
5
4
3
2
1 ppm
t
-Bu
1
H NMR
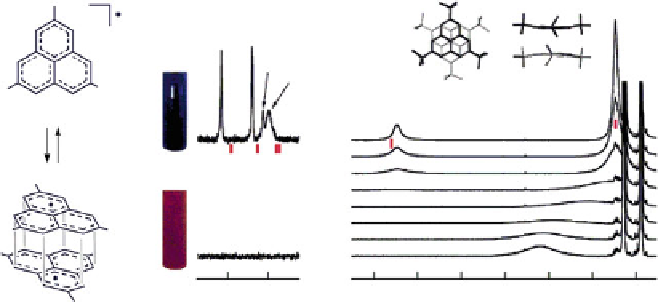
Fig. 4 (a) Schematic representation of the monoradical-
-dimer equilibrium for 10;(b) changes
in color,
13
C and
1
H NMR spectra in varied temperatures [
11
]. Reprinted with permission from
[
11
]. Copyright 2006 American Chemical Society
p
interaction (2
J
/
k
B
¼
2,000 K) from magnetic susceptibility measurement. This
result showed that the
-dimer was spin-singlet in the ground state.
The nature of phenalenyl
p
-dimer was later investigated by Kochi et al
.
in more
detail from both theoretical and experimental viewpoints [
8
-
10
]. Interestingly, the
same dimeric behavior was found in solution as that in crystal based on spectro-
scopic study, especially at lower temperature, leading to the thermochromic phe-
nomenon [
11
]. In other words, the red purple solution of 10 gradually turned blue
upon cooling, which is in accordance with the increase of absorbance in the
530-670 nm region, indicating the formation of dimer. More definite evidence
was provided by low temperature
1
H and
13
C NMR measurements. In the
1
H
spectrum at higher temperature the aromatic signals were broadened and the
tert
-
butyl signals were shifted downfield due to the effect of radical spin, whereas at
lower temperature, the diamagnetic dimer became the dominant species, so the
aromatic peak became sharp and the
tert
-butyl peak shifted to the normal region.
The
13
C NMR spectrum gave similar observations in the aromatic region due to the
presence of paramagnetic species. The molecular weight of the
p
-dimer was also
detected by cold-spray ionization mass spectrometry (CSI-MS) which allows
substances ionized at lower temperatures, and this result represented the first
detection of a radical dimer with NMR and MS techniques (Fig.
4
).
Phenalenyl radical derivatives with other substituents in the periphery, such as
alkoxy [
12
], hydroxyl, amino [
13
,
14
], and N-S-N groups [
15
], have been prepared
and studied. Among them, an interesting example was a perchlorophenalenyl
radical 16 with all
p
-positions substituted by chlorine atom. The
molecule was firstly prepared by Haddon et al. in 1987 [
16
] and the X-ray crystal
structure was obtained in 2001 [
17
]. The synthetic route is depicted in Scheme
2
,
chlorination of acenaphthene 11 affording a mixture of perchloroacenaphthene 12
and perchloroacenaphthylene 13, and conversion of 13 from 12 can be realized
a
-positions and
b