Chemistry Reference
In-Depth Information
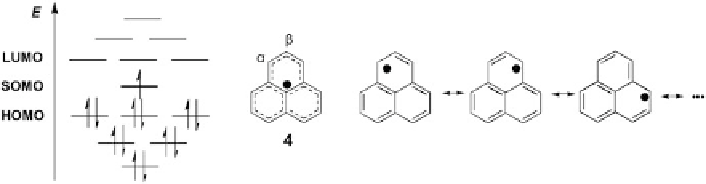
Fig. 3 Molecular orbital diagram and resonance structures of phenalenyl radical 4
come up with many methods to stabilize these systems, including steric protection,
incorporation of heteroatoms, and fusion with other aromatic skeletons. In addition,
it is quite exciting to note that real applications have already been realized in some
fields. In the following section we will summarize the recent advances in open-shell
PAHs with triangular shapes, including systems based on the phenalenyl radical
and the triangulene triplet diradical.
2.1 Phenalenyl-Based Open-Shell Systems
2.1.1 Phenalenyl-Based Monoradicals
The phenalenyl radical 4 represents the most fundamental and widely explored
member in this family. Unlike typical stable neutral radicals such as TEMPO and
a
-nitronylnitroxide derivatives with a spin-localized nature, 4 is characterized by a
planar, rigid structure with the spin spread over the whole molecular skeleton
(Fig.
3
). The resonance structures of 4 show that the spin density is predominantly
at its
a
b
positions) is much
smaller, which can be explained by the spin polarization effect. In addition, 4
exhibits a high amphoteric redox ability with thermodynamically stable cation,
neutral radical and anion species. All of these features of 4 lead to new insights in
the field of physical chemistry.
The first study of 4 dated back to the 1950s and it was found to be so reactive that
it can only be handled in solution under inert conditions [
5
]. Limited by the kinetic
instability of 4 caused by immediate intermolecular
positions, while the spin at the peripheral positions (
-bond formation and oxida-
tion by air, phenalenyl chemistry in the past half century has mainly been
performed in degassed solutions and under sealed conditions. Therefore, new
synthetic approaches and stabilization methods are badly needed to enrich
phenalenyl chemistry and to understand its properties in the solid state and crystal-
line phase.
The pioneering work of stabilizing phenalenyl radicals took advantage of
introducing primary alkyl groups, electron-donating and electron-withdrawing
groups [
6
]. Although isolation in the solid phase was not achieved, these systems
s