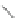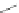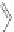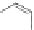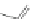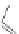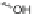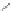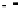Chemistry Reference
In-Depth Information
Scheme 48 Synthesis of 5,8-dimesityl [2,1-
c
]IF 175 (Fix, Deal, and Haley, 2012, unpublished
work)
Scheme 49 Lithium-induced cyclization to form dihydro [2,1-
c
]IF 178 [
96
]
IFs. The absorption spectrum of 175 displayed a broad region of lower energy
transitions ranging from approximately 500 nm to nearly 800 nm with the local
l
max
at 601 nm. Interestingly, and in contrast to the [1,2-
b
]IFs, cyclic voltammetry data
showed that arylation/full conjugation in 175 lead to a LUMO that is destabilized
with respect to that of parent [2,1-
c
]IF dione 149 (LUMO energies of 3.59 eV and
3.78 eV, respectively). X-Ray crystallographic analysis confirmed the structure
175, which showed a subtle helical twist to avoid steric interaction of the hydrogen
atoms located on carbons 1 and 12. Work on further derivatization of this scaffold is
ongoing (Scheme
48
).
6.3 Other Indeno[2,1-
c
]fluorenes
Youngs and Tessier synthesized one of the few [2,1-
c
]IF derivatives not
already discussed [
96
]. Treatment of tribenzocyclotriyne 176 with 4 equiv.
n
-BuLi collapsed the annulene core to furnish the [2,1-
c
]IF dianion 177; subsequent
quenching with MeOH as the proton source afforded the benzo-fused dihydro-IF
178 (Scheme
49
).
7 Conclusions
Unsurprisingly, given their interesting electronic properties, indenofluorenes, like
many PAHs, are experiencing a considerable reemergence into the current litera-
ture. While recent attention has mostly pertained to the [1,2-
b
] isomer, it is