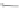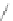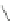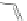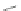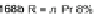Chemistry Reference
In-Depth Information
Scheme 46 Synthesis of dimerized [1,2-
a
]IFs 168a,b and spiro-fused [2,1-
c
]IFs 169a,b [
94
]
Scheme 47 Preparation of 6-isobutyl [2,1-
c
]IF dione 173 [
95
]
[
94
]. In the reaction that formed 168a, spiro-fused [2,1-
c
]IF derivative 169a was not
detected, but in the reaction that formed 168b, highly strained 169b was the major
product.
Isobutyl [2,1-
c
]IF dione 173 was isolated by Hilt and coworkers via a cobalt-
mediated [2+2+2] cyclization with 2 equiv. ethyl 3-phenylpropiolate (170) and
4-methylpentene (171) that gave terphenyl diester 172 in 86% yield (Scheme
47
)
[
95
]. Reaction of 172 with an excess of FeCl
3
cyclized and aromatized the central
ring to afford 173.
6.2 Fully-Conjugated Indeno[2,1-
c
]fluorenes
The first example of a fully-conjugated [2,1-
c
]IF (175) was recently realized by the
Haley group (Fix, Deal, and Haley, 2012, unpublished work). Similar to earlier
syntheses by Haley and Tobe, addition of mesityl anion to dione 149 gave 174 as a
mixture of stereoisomers. SnCl
2
treatment in the succeeding step gave 175 as a
green solid, as opposed to the magenta hue common to the fully-conjugated [1,2-
b
]