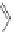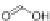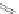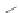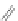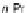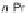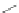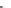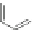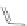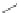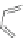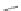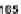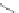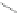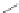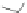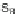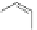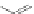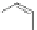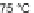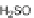Chemistry Reference
In-Depth Information
Scheme 43 Alternate synthesis to 149 [
92
]
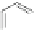
Scheme 44 Preparation of fused [2,1-
c
]IF diones 159-161 [
93
]
Scheme 45 Preparation of 6,7,-di-
n
-propyl [2,1-
c
]IF dione 165 [
94
]
A combination of bromination and elimination reactions on 159 resulted in an
unsaturation to ethene- and ethyne-bridged IFs 160 and 161, respectively.
Wang's group achieved substitution at the 6- and 7-positions of the [2,1-
c
] core
by reacting cyclopentadienone 162 with 4-octyne (163) in a Diels-Alder reaction to
form terphenyl diester 164 (Scheme
45
)[
94
]. Cyclization with concentrated sulfu-
ric acid gave 6,7-di-
n
-propyl [2,1-
c
]IF dione 165 in 79% yield. These results stand
in contrast to the observations in Scheme
11
where use of either phenylacetylene or
diphenylacetylene gave the corresponding [1,2-
b
] isomer in high yield.
In addition, the same group found that reaction of [2,1-
c
]IFs 165 and 166 with an
acetylide nucleophile gave diols 167a,b; subsequent treatment with SOCl
2
induced
a cascade cyclization forming [1,2-
a
]IF dimers 168a,b in low yield (Scheme
46
)