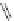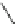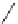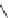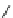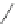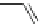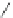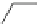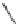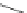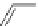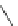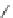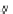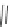Chemistry Reference
In-Depth Information
Cl
Cl
PdCl
2
(PCy
3
)
2
DBU, DMF
C
6
H
5
I
Pd(OAc)
2
AgOAc
Cl
Cl
31%
Ar
Ar
60%
R
R
R
R
151
150
Cl
Cl
RhCl(PPh
3
)
3
R
R
99%
PdCl
2
(PCy
3
)
2
DBU, NMP
87
Cl
Cl
Cl
20%
Ar =
R
R
R
R
153
Cl
152
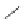
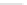
Scheme 47 Synthesis of mixed buckybowls 151 and 153 [
162
,
164
]
X
X=H,1250ºC
0.6%
X=Cl,1100ºC
25
-
27%
X
X
154
155
Scheme 48 Synthesis of circumtrindene (155)[
165
,
166
]
Moreover, a new methylene-bridged buckybowl 153 (R
¼
H) was obtained from
fluoranthene 152 (R
H) through benzylic and aryl C-H bond activation
[
163
]. According to the crystallographic analysis, both buckybowls 151 (R
¼
H)
and 153 (R
¼
H) have very high POAV pyramidalization angles and deep bowl depths
[
162
,
164
]. The maximum POAV pyramidalization angles for 151 and 153 was
observed at carbon atoms in the central five-membered ring, both with the value of
12.8
.In151, bowl depths measured from the corannulene and sumanene cores were
1.24 and 1.48
¼
, respectively. These values are significantly larger than those for the
parent compounds corannulene (0.87
Å
). It should be noted
that the highly curved structures for 151 and 153 correspond to their high bowl-to-
bowl inversion barriers. The inversion barrier of the former was determined compu-
tationally to be 124.3 kcal/mol. The cyclopenta-annulation increases the bowl depth
Å
) and sumanene (1.11
Å