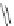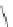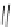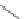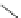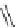Chemistry Reference
In-Depth Information
Scheme 46 Synthesis of
buckybowl 149 [
25
]
Br
Br
FVP
1050 ºC
7%
Br
149
Br
148
was investigated [
160
]. They form the supramolecular complex with a comparable
binding constant to that for the association of 147 with C
60
.
4 Mixed Type Buckybowls
A buckybowl containing both corannulenyl and sumanenyl fragments is described
as a mixed buckybowl in this context. Pentaindenocorannulene (104) is one such
example.
4.1 Acenaphtho[3,2,1,8-ijlkm]diindeno[4,3,2,1-
cdef:1
0
,2
0
,3
0
,4
0
-pqra]triphenylene
Scheme
46
presents an unexpected formation of buckybowl 149 from corannulene
derivative 148, which was prepared by a protocol similar to Method C in Scheme
5
[
25
]. Under FVP conditions, 148 underwent cyclization to give hydrocarbon 149
through the formation of three C-C bonds. The key step in the cyclization should be
1,2-shift of the hydrogen atom of the rim radical, generated by the rupture of C-Br
bond one at a time, for the formation of five-membered rings.
4.2 Acenaphth[3,2,1,8-fghij]-as-indaceno[3,2,1,8,7,6-
pqrstuv]picene
Buckybowl 151 (R
H) was first prepared by an inefficient route in which 7,12-bis-
(2-bromophenyl)benzo[
k
]fluoranthene was cyclized under FVP conditions at
1,100
C[
161
]. Later on, a new synthetic approach in solution phase made 151
easily accessible. The first step of the synthesis was Pd-catalyzed annellation of
1,8-bis(arylethynyl)naphthalene 87 (R
¼
H) with iodobenzene to give benzo[
k
]
fluoranthene 150 (Scheme
47
)[
28
]. Cyclization of 150 was conducted with a
mixture of DBU and Pd(PCy
3
)
2
Cl
2
to generate 151 (R
¼
¼
H) in 31% yield [
162
].