Information Technology Reference
In-Depth Information
(a)
1.8
(b)
2.5
1.6
2
1.4
1.2
1.5
1
0.8
1
0.6
0.4
0.5
0.2
0
0
0
100
200
300
400
500
600
700
800
0
200
400
600
800
t(s)
t(s)
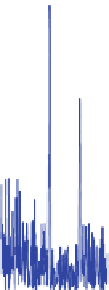
Fig. 11 Relative error for the two proposed methods a with Chiu, and b with DBSCAN
6 Experimental Example: A Semi-batch Reactor
6.1 Process Description
The olive oil esteri
cation reactor produces ester with a very high added value
which is used in
ca-
tion reaction between vegetable olive oil with free fatty acid and alcohol, producing
ester, is given by the following equation:
fine chemical industry such as cosmetic products. The esteri
Acid
þ
Alcohol
$
Ester
þ
Water
:
ð
38
Þ
The ratio of the alcohol to acid represents the main factor of this reaction because
the esteri
cation reaction is an equilibrium reaction i.e. the reaction products, water
and ester, are formed when equilibrium is reached. In addition, the yield of ester
may be increased if water is removed from the reaction. The removal of water is
achieved by the vaporisation technique while avoiding the boiling of the alcohol. In
fact, we have used an alcohol (1-butanol), characterized by a boiling temperature of
118
C).
In addition, the boiling temperatures of the fatty acid (oleic acid) and the ester are
close to 300
°
C which is greater than the boiling temperature of the water (close to 100
°
C. Therefore, the boiling point of water may be provided by a
temperature slightly greater than 100
°
C.
The block diagram of the process is shown in Fig.
12
. It is constituted essentially
°
of:
A reactor with double-jackets: It has a cylindrical shape manufactured in
stainless steel. It is equipped with a bottom valve for emptying the product, an
agitator, an ori
ce introducing the reactants, a sensor of the reaction mixture
temperature, a pressure sensor and an ori
ce for the condenser. The double-


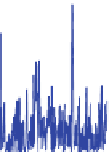




























































Search WWH ::

Custom Search