Chemistry Reference
In-Depth Information
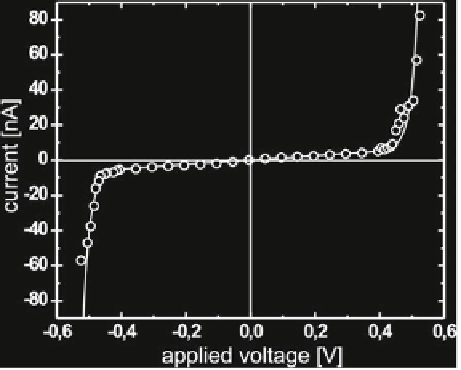
Fig. 2.12
Current/voltage
characteristic of a mono-olein
membrane. Aside from an
ohmic central part, there is a
pronounced rise in the current
at a threshold voltage of about
500mV. This is attributed
to reversible electroporation.
The solid curve corresponds
to what would be expected
theoretically in that case
liquid necks within themembrane [
28
,
29
]. Also, it is known fromstudies on cells that
pore formation is favoured when the electric pulses are relative short and typically
below 1 ms [
30
]. The rate of creation of (transient) nano-pores due to the applied
electric field is believed to scale as exp
U
2
(
/
kT
)
, where
U
is the applied voltage
[
29
]. Accordingly, the solid curve has the form
I
0
sinh
bU
3
|
I
=
aU
+
(2.5)
U
|
where
a
accounts for impurity-induced ohmic conductance. For the constant
b
,we
obtain 49 kT
V
2
, which is a reasonable value for bilayer membranes [
29
]. The sharp
rise of the current at the critical voltage of about 500mV shows that the membrane
can be used as a voltage stabilization device, similar to a Zener diode, without further
manipulation.
/
2.4 Summary and Outlook
Self assembled surfactant bilayer networks in microfluidic channels may provide
a crucial first step towards complex dynamical functions comprising nanoscale or
molecular units. More specifically, native surfactant bilayers already offer a range
of different electrical behaviour that can be exploited to create wet circuitry. The
stability of these objects in micro-fluidic systems is quite encouraging, both in static
and in dynamic settings. Their employment as externally controlled scaffolds for
synthetic functional molecular units thus appears feasible. The peculiar permeation
properties of bilayer membranes for messenger molecules, such as those occuring