Chemistry Reference
In-Depth Information
While the capacitive signal is unambiguously due to the membrane capacitance
and corresponds to the specific capacitance of about 0
cm
2
(c.f. Fig.
2.6
), the
ohmic resistance is masked by the resistance of the ionic conductance of the sur-
rounding aqueous solution. Comparing the traces obtained with the configurations
displayed if Fig.
2.9
a, b, we obtain the 'pure' ohmic characteristic of a single mem-
brane, as displayed in Fig.
2.10
b. This conduction is probably due to ionic impurities
which are sufficiently lipophilic to cross the membrane [
25
] in a thermally activated
process.
Next we want to demonstrate that the capability of membranes to incorporate
active components, as it is well known from experiments in membrane physiology
[
22
,
26
], also pertains to the micro-fluidic setting. We have therefore added grami-
cidin ion pores to the liquid phase in the device, in order to see whether the presence
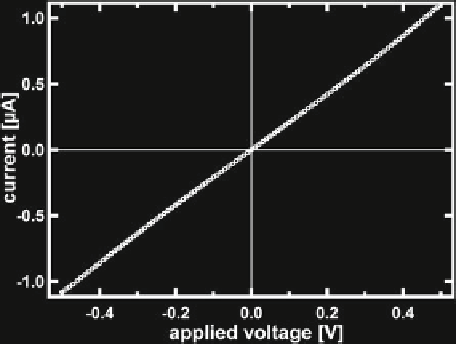
of the channel walls might hamper their performance noticeably. Figure
2.11
shows
the current-voltage relationship of a membrane doped with gramicidin A ion pores
(Sigma-Aldrich). The gramicidin is added to the aqueous phase at a concentration of
1
.
µ
/
7
F
M. Clearly, there is now a dramatically increased conductance of themembrane,
which we attribute to the ionic conductivity of the pores. From the unit conductance
of the gramicidin A ion pore [
27
], we estimate the number of pores in our membrane
to be in the order of 10
6
. Since the membrane is now significantly conducting as
compared with the native state, voltages of 500mV (and even up to 1V) can easily
be applied without rupturing the membrane. In the native state, we found that applied
voltages beyond roughly 300mV ruptured the membrane.
Along with the applied voltage, the width of the applied voltage pulse is also
crucial in studying the current/voltage characteristic of a membrane. The result of
such an experiment on a native membrane similar to the one described above is
displayed in Fig.
2.12
. In comparion with the previous experiment (pulse width of
10ms), here the applied pulse is much shorter and is of 1 ms duration. We observe a
clearly defined threshold voltage above which the current rises sharply. We interpret
this as reversible electroporation due to electric-field induced formation of nano-scale
.
1
µ
Fig. 2.11
Current/voltage
characteristic of a mono-
olein membrane doped with
Gramicidin A ion pores