Chemistry Reference
In-Depth Information
5.4 PROPYLENE
Like ethylene, propylene is produced by the cracking of hydrocarbons. The
major use of propylene is to make polypropylene but it is also a feedstock to
manufacture other industrial chemicals, including propylene glycol, acrylic
acid, propylene oxide, cumene, and isopropyl alcohol. Propylene oxide is
made by the oxidation of propylene, and hydrolysis of propylene oxide gives
propylene glycol. The alkylation of benzene with propylene gives isopropyl
benzene, more commonly called cumene. Note that the product is not n-propyl
benzene. This is because the intermediate is the more stable secondary
carbocation which results in isopropyl benzene.
H
+
H
3
C
H
H
CC
H
H
H
H
+
−
H
+
Cumene
Cumene is converted to phenol and acetone. The reaction involves the
oxidation of cumene to cumene hydroperoxide. This can be done in a
radical process by passing air through cumene. In a second step, the cumene
hydroperoxide is then treated with acidic water to form phenol and acetone
in a 1:1 molar ratio.
+
+
+
H
+
,
H
+
−
+
+
+
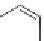
Phenol and acetone can be reacted together in a 2:1 molar ratio to make
bisphenol-A (BPA). This is an electrophilic aromatic substitution reaction
with the electron-rich phenol ring and acetone as the electrophile.




































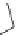




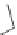



















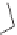





























Search WWH ::

Custom Search