Chemistry Reference
In-Depth Information
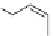
OH
H
H
H
O
H
3
C
CH
3
O
O
H
+
O
H
H
H
+
,
−
H
2
O
O
O
H
O
O
H
BPA
H
Two molecules of phenol are used for each mole of acetone. Because
phenol and acetone are produced in a 1:1 molar ratio and consumed in a 2:1
molar ratio, there is an excess of acetone. Therefore acetone is often a glut
on the market and is a generally inexpensive solvent. The BPA is used to
make various polymers, including polycarbonate, many epoxy resins, and
some phenolic resins. BPA is used in many polymers that have food contact
and BPA can be present in minute amounts. Examples of these applications
include some plastic containers, bottles, and can linings. Therefore, there
is some concern about possible health effects. It continues to be a matter of
study, but one review of the research suggests that current exposure levels
are safe [14]. Phenol is also used to make phenol resins and as a feedstock in
the manufacture of caprolactam, the monomer for nylon 6.
Acrylic acid represents a 4.5 million metric ton per year global market
with BASF, Dow, and Arkema being major manufacturers [15]. Acrylic acid
is manufactured by the oxidation of propylene. The reaction is a two-step
process with oxidation to acrolein followed by further oxidation of the
aldehyde to the carboxylic acid. Acrolein is a raw material for cosmetics,
flavors, and pharmaceuticals. The major use of acrylic acid is as a monomer
to make acrylic acid polymers and conversion to acrylate esters which are
also used to make polymers.
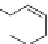
O
2
−
H
2
O
O
2
O
H
H
O
O
Propylene
Acrolein
Acrylic acid
Although most acrylic acid is produced from propylene, there are other
routes including processes that begin with ethylene oxide or acetylene. One
process being developed independently by Arkema and Nippon Shokubai
[16] is based upon glycerol as a starting material. Glycerol (also known as
glycerin or glycerine) is a byproduct of biodiesel formation. A vegetable oil
is transesterified with methanol to give the methyl ester, used as biodiesel, and
glycerol. The R groups vary with different vegetable oils but are typically 11
to 17 carbons in length. The glycerol can be converted to biobased acrylic
acid either directly or via acrolein.






























































Search WWH ::

Custom Search