Chemistry Reference
In-Depth Information
O
S
NHCH
3
Duloxetine
O
O
OH
OH
O
S
S
S
O
Cl
Cl
+
Cl
CALB
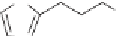
Racemic alcohol
The precursor alcohol is formed as a racemate and then reacted with vinyl
butanoate to 50% conversion in the presence of catalytic
Canidida antarc-
tica
Lipase B (CALB). The undesired R alcohol esterifies faster. After 50%
conversion, it has formed the ester and the desired S alcohol remains [43] for
subsequent conversion to duloxetine.
One method that avoids the need for asymmetric synthesis or separation
of the racemic mixture is to begin with an enantiomerically pure starting
material and maintain the chirality throughout the subsequent synthetic
steps. This is sometimes called a chiral pool synthesis and is especially
useful when the chiral starting material is an inexpensive naturally occurring
chemical.
Some drugs can undergo in-vivo chiral inversion. For example, only
the S enantiomer of ibuprofen is active. It is over 100-fold more potent as
an inhibitor of cyclooxygenase I than the R enantiomer of ibuprofen [44].
However in the body, the R enantiomer undergoes chiral inversion into
the active S enantiomer. In the case of ibuprofen, the S enantiomer is not
converted to the R. The inversion is unidirectional and a single enantiomer is
formed. However, with other chiral actives, in-vivo racemization can occur.
One example is thalidomide. This drug was prescribed in Europe for pregnant
women as a sleep aid and to counter morning sickness. Its use resulted in
many horrible birth defects in the 1960s. Later studies suggested that the
R-enantiomer had the desired therapeutic benefit and that the S-enantiomer
was mutagenic [45]. However, the R-enantiomer is not suitable for this appli-
cation because it racemizes in-vivo to form the undesirable S-enantiomer.
Racemic thalidomide is used for other therapies. It is the drug of choice to
treat leprosy, albeit under conditions carefully controlled by physicians. It
has also been approved for the treatment of multiple myeloma, a form of
cancer [46].















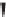









Search WWH ::

Custom Search