Chemistry Reference
In-Depth Information
HO
CO
2
H
Mandelic acid
Asymmetric synthesis can also sometimes be done by biocatalysis. This
has been used in large-scale syntheses for years. For example, the Reich-
stein process for the synthesis of vitamin C, established in 1933 and still in
use, involves the biotransformation of sorbitol to L-sorbose [41]. L-carnitine
is used as a thyroid inhibitor. It is prepared by enzymatic hydroxylation of
4-butyrobetaine [42].
H
3
C
H
3
C
O
OH
O
H
3
C
H
3
C
N
N
O
O
H
3
C
H
3
C
L-carnitine
4-butyrobetaine
Of the methods to prepare single enantiomers, resolution by preparation
of the diastereomers from the racemic mixture and then separation of the
diastereomers followed by regeneration of the single enantiomer might be the
most common. If the racemic mixture has either acidic or basic functionality,
this can be accomplished by salt formation. For example, in the synthesis of
levothyroxine, used to treat hypothyroidism, the chiral center is resolved at
an intermediate stage. The intermediate is a racemic mixture and has a car-
boxylic acid functionality. When the racemic mixture is treated with a chiral
amine to form the ammonium carboxylate, two diastereomers are formed.
These can be separated and then the carboxylic acid of the single enantiomer
regenerated by treatment with acid.
I
I
O
OH
I
O
O
N
H
2
H
HO
I
N
HO
I
CO
2
H
Racemic intermediate
H
O
I
Levothyroxine
Asymmetric synthesis is also common. Another technique is kinetic res-
olution. Kinetic resolution relies upon a difference in reactivity between the
two enantiomers. For example this technique can be used in the synthesis
of duloxetine. Duloxetine is marketed as the hydrochloride salt under the
tradename Cymbalta
®
as an antidepressant. It is a selective serotonin and
norepinephrine reuptake inhibitor. It is the S enantiomer which is used.









































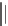


















Search WWH ::

Custom Search