Chemistry Reference
In-Depth Information
Another possible way for a growing chain to terminate is if the radical
abstracts a hydrogen atom from another growing chain. This termination
mechanism is called disproportionation.
H
H
H
Pol
Pol
Pol
Pol
Disproportionati
o
n
+
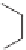
Last, a chain can terminate by abstracting a hydrogen atom from a chain
transfer agent. The chain transfer agent can be present as an unwanted impu-
rity. Often a chain transfer agent is deliberately added to a polymerization
to control molecular weight by terminating the reaction. The reaction is
illustrated with butyl mercaptan.
S
H
Pol
Pol
Chain transfer
+
S
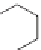
In an anionic polymerization, the chain transfer agent is anything that can
donate a proton. It is illustrated below with isopropanol as the chain transfer
agent.
O
H
Pol
Pol
O
Chain transfer
+
In a living polymerization, there is no chain transfer agent and the
polymerization does not terminate. Living polymerizations tend to have
a narrower molecular weight distribution and the molecular weight is a
function of the amount of added monomer per amount of initiator. For a
given amount of initiator, molecular weight increases with increasing amount
of monomer. Anionic living polymerizations were discovered in the 1950s
when researchers [18] observed that the viscosity of the polystyrene solution
continued to increase with addition of more styrene monomer. This enabled
anionic block polymers of styrene with rubbers such as butadiene or iso-
prene. These polymers were commercialized by Shell Chemical Company as
Kraton
®
polymers. For example, styrene can be polymerized in the absence




































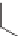






















Search WWH ::

Custom Search