Chemistry Reference
In-Depth Information
the Greek meaning to transpose or changing places. The olefin metathesis
reaction is widely used in organic synthesis. Yves Chauvin, Robert Grubbs,
and Richard Schrock were awarded the 2005 Nobel Prize in Chemistry for
their work in this area. Simply viewed, olefin metathesis is an olefin exchange
as exemplified by the general olefins a
=
bandc
=
dorbythereactionof
2,3-dimethyl-2-butene with 3,4-diethyl-3-hexene.
a
c
ac
+
b
d
bd
H
3
C
CH
3
H
3
CH
2
C
CH
2
CH
3
H
3
CH
2
C
CH
2
CH
3
+
H
3
C
CH
3
H
3
CH
2
C
CH
2
CH
3
H
3
C
CH
3
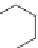
If the olefin is contained within a ring, preferably one with ring strain, it
can react with itself by ring opening and the newly formed olefin is a polymer.
This is exemplified by the ring-opening polymerization of norbornene.
x
Note that although most olefin polymerizations “consume” the double
bond, here the unsaturation is maintained. The polymer repeat unit in
polyethylene or polystyrene does not include an olefin, but there is a repeat
olefin in ROMP polymers.
Another polymerization type is living polymerizations. In a living
polymerization, the polymerization proceeds to full conversion with further
monomer addition leading to continued polymerization. This means that the
growing polymer chain does not terminate, but remains reactive or alive.
Polymerizations can terminate by chain transfer reactions or in the case of
radical polymerization by combination or disproportionation.
Consider the radical polymerization of styrene. Two growing polymer
chains can combine. This terminates the polymerization.
Pol
Pol
Pol
Pol
Combination
Pol = growing polymer chain

























































Search WWH ::

Custom Search