Chemistry Reference
In-Depth Information
of a chain transfer agent until the styrene monomer is consumed. Butadiene
can then be added and a rubber block added to make SB (styrene butadiene)
polymer. Then more styrene can be added to the living polymerization to
make SBS triblock copolymer. Lastly, the polymerization can be terminated
by addition of a chain transfer agent.
Li
+
CH
2
x
CH
2
CH
=
CHCH
2
CH
2
CH
=
CHCH
2
y
+
x
m
CH
2
CH
=
CHCH
2
CH
2
CH
=
CHCH
2
y
+
m
CH
2
CH
=
CHCH
2
n
ROH
x
m
CH
2
CH
=
CHCH
2
CH
2
n
x
m
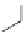
Three decades after the discovery of living anionic polymerizations, living
carbocationic polymerizations were discovered [19]. Isobutylene was poly-
merized in the presence of cumyl acetate and boron trichloride. The molecular
weight increased with increasing amount of isobutylene. In a typical cationic
polymerization, the reaction is initiated by protonation of the alkene to give
a carbocation. Termination happens by elimination to form an alkene.
In a normal (non-living) cationic polymerization, an initiator provides a
cation source. This is illustrated below with a proton as the cation. The proton


























































































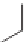


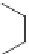











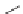
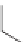































Search WWH ::

Custom Search