Biomedical Engineering Reference
In-Depth Information
thermochemical processing. The differential scanning calorimetry (DSC)
may be used to determine these parameters of a substance.
The unit essentially consists of two solid pans resting on top of hea-
ters. The sample to be tested is kept on one of the pans while the other is
kept empty (reference pan). A computer program accurately controls the
rate of temperature rise, and records the exact amount of heat supplied to
each pan. The amount of heat supplied to the reference pan is lower than
that supplied to the pan with the solid sample. An additional heat is
required exclusively to heat the sample to maintain the same rate of tem-
perature rise.
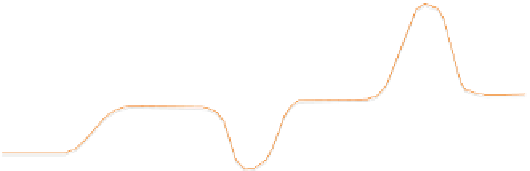
The difference in heat given out by two heaters to maintain an identical
temperature rise is plotted in the y-axis while the temperature is plotted in
the x-axis (
Figure 13.3
). Different temperatures, like glass transition tempera-
ture T
g
, crystallization temperature T
c
, and melting point T
m
, are denoted in
the graph shown, which is the typical result from a DSC experiment.
One can determine the heat capacity C
p
by dividing the heat flow-rate by
the temperature change rate.
C
p
5
ð
dQ
=
dt
Þ
ð
dT
=
dt
Þ
When the sample reaches its glass transition temperature, the heat capac-
ity does not remain constant anymore. It requires more heat to raise the tem-
perature. Because of the change in heat capacity, the heat input rate to the
sample rises shifting the graph upward. Beyond this range, the graph flattens.
The glass
transition temperature (T
g
) at
the middle of
the incline
(
Figure 13.3
).
Certain molecules crystallize at a temperature T
c
. Since crystallization is
an exothermic process during which heat is released, this process can be
characterized by a dip in the plot.
T
g
T
c
T
m
Temperature (K)
FIGURE 13.3
Heat flow plot in a DSC.














Search WWH ::

Custom Search