Biomedical Engineering Reference
In-Depth Information
The bomb calorimeter consists of pressurized oxygen “bomb” (30 bar),
which houses the fuel. A 10 cm fuse wire connected to two electrodes is
kept in contact with the fuel inside the bomb. The oxygen bomb is placed in
a container filled with 2 l of deionized water. The temperature of the water
is measured by means of a precision thermocouple. A stirrer stirs the water
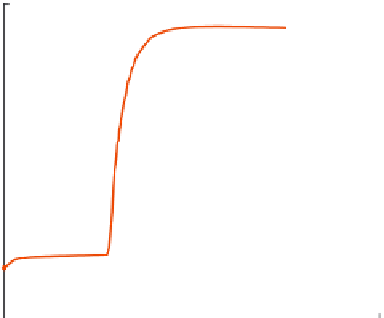
continuously. Initially, the temperature change would be small (
Figure 13.2
)
as the only heat generated would be from the stirring of the water molecules.
After the temperature is stabilized, the sample is fired, meaning a high volt-
age is sent across the electrodes and through the fuse wire. The electric cur-
rent passing through the fuse wire would almost instantly ignite and combust
the fuel sample in oxygen. The water absorbs the heat, released by the com-
bustion of the sample, resulting in a sharp rise in the water temperature
(
Figure 13.2
). The temperature continues to rise for sometime before leveling
off. The water temperature is continuously recorded till the temperature read-
ings are stable. Knowing the heat capacity of the bomb calorimeter material,
water, and of the fuse wire, one can calculate the exact amount of heat
released by combustion of the sample.
By knowing the initial mass of the fuel sample, the heating value of the
sample can be calculated by dividing the heat released by the mass of the
sample. As the product of combustion is cooled below the condensation tem-
perature of water, this technique gives the HHV of the fuel.
13.3 DIFFERENTIAL SCANNING CALORIMETRY
Heat capacity, glass transition temperature, crystallization temperature, and
melting point are some important parameters of a fuel undergoing
26
25.5
25
24.5
24
23.5
23
0
500
1000
1500
2000
Time (s)
FIGURE 13.2
Temperature profile from a bomb calorimeter experiment.

















Search WWH ::

Custom Search