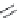Biomedical Engineering Reference
In-Depth Information
H
H
S
H
H
R O HC
C
CH
2
S
CH
3
CH
3
6
C
7
R O
NH
C
C
5
3
6
C
N
C
CH
2
R'
COONa
O
C
N
C
C
3
O
H
COOH
Penicillin
Cephalosporin
H
H
S
H
H
H
2
NC
C
CH
2
S
7
CH
3
CH
3
C
H
2
N
C
C
C
N
C
CH
2
OAc
6
O
C
COOH
C
N
C
H
O
COOH
6-Aminopenicillanic acid
6-APA
7-Aminopenicillanic acid
7-APA
S
S
N
N
N
O
O
O
Penam
Penem
Carbapenem
X
O
3
4
RCONH
N
NH
N
2
1
O SO
3
H
Monobactam
O
O
Azetidin-2-one
β-lactam
Clavam
FIGURE 25.1
b-Lactam structures.
producing bacteria. Penicillanic acid sulfone (sulbactam) and its prodrug pivsulbactam are also
inhibitors of b-lactamase and are combined with ampicillin (Figure 25.2).
25.2.1.4.2 Carbapenems
A new b-lactam antibiotic, thienamycin, was obtained in 1978 from cultures of
Streptomyces cattleya
by researchers at Merck Sharp and Dohme. The bicyclic system, with a double bond between
C
2
and C
3
, is called 2-carbapenem (Figure 25.1). Several related products were discovered, e.g.,
epithienamycins (isomers in hydroxyethyl side chain). Thienamycin is not stable and is used as the
N
-formimidoyl derivative (imipenem). Thienamycin and imipenem are prepared by total synthesis.
Imipenem and meropenem are broad-spectrum antibiotics. Another problem was discovered during
clinical studies, i.e., the cleavage of thienamycin by a dehydropeptidase present in the kidney. For
that reason, imipenem is associated with an inhibitor of that enzyme, cilastatin. Imipenem exhibits
a broad spectrum of activity and is resistant to most b-lactamases (Figure 25.2).
25.2.1.4.3 Penems
In 1977, Woodward described the i rst synthesis of a penem. This penem, which had a phenacetyl-
amido side chain like penicillin, had a rather low activity. The introduction of hydroxyethyl groups
in C
6
, like in the carbapenems, improved the potency signii cantly.