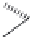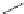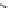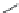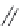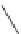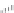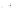Biomedical Engineering Reference
In-Depth Information
HO
CH
2
OH
H
H
H
H
O
H
3
C
H
S
2
NH
2
COOH
N
N
O
O
H
COOH
Thienamycin
Clavulanic acid
OH
CH
3
H
H
SO
2
CH
3
S
C
CH
3
COOH
H
CONMe
2
N
N
C
O
H
O
COOH
Meropenem
Sulbactam
HO
H
H
H
H
SO
2
CH
3
C
H
H
3
C
CH
3
COO
H
S
2
CH
2
NH
C
CH
2
O
C
C(CH
3
)
3
N
C
N
NH
2
O
O
O
COO
Imipenem
Pivsulbactam
NH
2
CH
2
S
CH
COONa
CH
2
HN
O
COONa
Cilastatin (Na)
FIGURE 25.2
Nonclassical b-lactams.
25.2.1.4.4 Monobactams
The term monobactam was coined by Sykes et al. (1981) to describe a novel group of bacterially
produced monocyclic b-lactams. A product SQ26.180 was isolated by Sykes from
Chromobacterium
violaceum
and a related product, sulfazecin was discovered in Japan by Imada et al. (1981) in cul-
tures of
Gluconobacter
sp., and
Pseudomonas
sp. The activity was improved by modii cation of
the amide side chain. The introduction of the cefotaxime side chain gave an interesting new drug,
aztreonam. It is very active against Gram-negative bacteria, including
Pseudomonas aeruginosa
(Figure 25.3).
25.2.2 B
ACITRACIN
Bacitracin is produced by a strain of
Bacillus subtilis
and was isolated in 1945 from the wound of a
patient called Tracy. The same product was discovered in Oxford in 1949, in the culture of
Bacillus
licheniformis
A5, and was called Ayi vin. Studies by Abraham et al. and by Craig et al. in New York
revealed that bacitracin (= ayi vin) was a mixture and it had a peptide structure. The structure of the
main component is given in Figure 25.3.