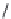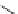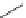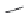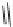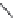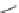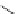Biomedical Engineering Reference
In-Depth Information
O
NH
2
O
H
3
C-NH
OH
OH
HN
OH
HN
OH
N
N
O
O
Nipecotic acid (
15.15
)
Guvacine (
15.16
)
exo
-THPO (
15.17
)
N
-Me-
exo
-THPO (
15.18
)
O
O
S
N
OH
N
OH
S
OH
N
S
N
S
O
N
-DPB-nipecotic acid (
15.19
)
Tiagabine (
15.20
)
EF-1502 (
15.21
)
FIGURE 15.4
Structures of some GABA transport inhibitors.
barrier and are potent anticonvulsants in animal models. Tiagabine (
15.20
), a structurally related
compound, is now marketed as an add-on therapeutic agent for the treatment of epilepsy.
A highly glia-selective compound was discovered based on the structure of
exo
-THPO (
15.17
),
where the monomethylated compound
N
-methyl-
exo
-THPO (
15.18
) proved to be the most selective
inhibitor for glial vs. neuronal GABA uptake reported yet.
EF-1502 (
15.21
), developed as a hybrid of
exo
-THPO and Tiagabine, has similar potency at
GAT-1 and BGT-1. An
in vivo
study of the anticonvulsant properties of the compound revealed a
synergistic effect between EF-1502 and GAT-1-selective inhibitors, indicating a possible role for
BGT-1 as a therapeutic target (Figure 15.4).
15.5 GABA RECEPTORS AND THEIR LIGANDS
GABA exerts its effects on the CNS via two different types of receptors: the ionotropic GABA
A
and GABA
C
receptors, mediating the fast synaptic transmission and the G-protein-coupled GABA
B
receptors, mediating the slower responses to GABA via coupling to second messenger cascades.
15.5.1 I
ONOTROPIC
GABA R
ECEPTORS
The ionotropic GABA receptors belong to a superfamily of ligand-gated ion channels (Cys-loop
receptors) that also includes the nicotinic acetylcholine, the glycine, and the serotonin (5-HT
3
)
receptors (see Chapter 12). Whether the GABA
C
receptor is a subgroup of the GABA
A
receptors or
a distinct group of GABA receptors is still a matter of debate. The GABA
A
receptors are widely dis-
tributed in the CNS and involved in a wide variety of CNS functions, whereas the GABA
C
receptors
predominantly are expressed in the retina and primarily implicated in visual processing. However,
GABA
C
receptors have also been identii ed in some CNS regions, where they have been proposed
to be involved in processes connected with sleep and cognition processes.
The ionotropic GABA receptors are transmembrane protein complexes composed of i ve sub-
units. So far, 19 human GABA receptor subunits have been identii ed, and they have been classii ed
into
ρ
1-3
subunit classes (Figure 15.5). Each of the subunits consists of an
amino-terminal domain and a transmembrane region formed by four transmembrane
α
1-6
,
β
1-3
,
γ
1-3
,
δ
,
ε
,
π
,
θ
and
-helices con-
nected by intra- and extracellular loops. In the pentameric GABA receptor complex, the orthosteric
site (i.e., the binding site for the endogenous ligand GABA) is formed at the interface between the
terminal domains of two subunits, whereas the transmembrane regions of the subunits form the ion
channel pore through which chloride ions can enter the cell upon activation. The GABA
A
receptors
α