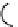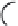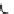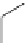Biomedical Engineering Reference
In-Depth Information
O
O
-
-
O
O
Enz
Enz
Enz
+
H
+
+
+
H
2
N
H
NH
2
NH
NH
HN
+
OH
OH
OH
O
H
3
N
P
P
P
+
-
O
N
N
N
+
+
H
H
H
(
15.8
)
(
15.3
)
(
15.9
)
(
15.10
)
O
O
-
-
O
O
-
X
X
Enz
+
+
Enz
H
3
N
NH
O
NH
-
OH
OH
OH
O
P
P
+
P
Inactivation
H
2
O
N
N
N
+
+
+
O
H
H
H
(
15.14
)
(
15.11
)
(
15.12
)
(
15.13
)
FIGURE 15.3
Proposed inactivation mechanism of GABA-AT by Vigabatrin (
15.3
). The cofactor PLP
and an amino group from a lysine residue in GABA-AT (Enz) form a Schiff base (
15.8
), which reacts with
Vigabatrin and eventually leads to inactivation of GABA-AT.
dil uoromethylene analog
15.7
(Figure 15.2), is reported as a markedly more potent inactivator of
GABA-AT than Vigabatrin.
15.4 GABA TR ANSPORT
The GA BA transpor ters belong to the fam ily of Na
+
/Cl
−
dependent transpor ters (SLC- 6 gene fam ily)
that also include transporters for the neurotransmitters dopamine, serotonin, norepinephrine, and
glycine (see Chapter 14). Four subtypes of GABA transporters have been identii ed in the mama-
lian CNS. For rat and human GABA transporters, the nomenclature is GAT-1, betaine/GABA-
transporter-1 (BGT-1), GAT-2, and GAT-3.
15.4.1 I
NHIBITORS
OF
GABA T
RANSPORT
The pharmacological inhibition of GABA transporters constitutes an attractive approach to increase
the overall GABA neurotransmission. A selective blockade of glial uptake is believed to be optimal,
as this will ensure an elevation of the GABA level in the presynaptic nerve terminals.
Nipecotic acid (
15.15
) and guvacine (
15.16
), competitive inhibitors and substrates for the GABA
uptake, have been important lead structures for the development of a large number of lipophilic
GABA uptake inhibitors. Introduction of a lipophilic moiety, such as 4,4-diphenyl-3-butenyl (DPB),
on the nitrogen atom led to
N
-DPB-nipecotic acid (
15.19
) and related analogs, which are markedly
more potent than the parent amino acids. These lipophilic compounds are able to cross the blood-brain