Biology Reference
In-Depth Information
Actually, our ultimate goal was to obtain thermotropic liquid
crystals of the discotic type through G-quartets stacking: funnily
enough, we have observed many things on these derivatives, as will
be described below, but no discotic liquid crystals so far.
Of course, a requirement for the synthesis of these at-that-time-
unknown LGs was that the H-bond donors and acceptors on the
nucleobase were preserved while long aliphatic tails (in our case)
were introduced in the sugar moiety. This was originally achieved
through a sequence of orthogonal protection and deprotection steps,
where standard nucleotide chemistry procedures were integrated
with some typical peptide chemistry, as depicted in Scheme 4.1.
O
O
O
O
H
N
O
H
H
H
N
N
N
N
N
N
N
i
1) ii
2) iii
1) iv
2) v
R
O
N
N
NH
2
HO
N
Me
3
SiO
N
HO
N
N
NH
2
N
NH
2
N
NH
Fmoc
O
O
O
O
R
O
HO
Me
3
SiO
HO
O
Scheme 4.1
General synthesis of LG esters: i) Me
SiCl/pyr; ii) Fmoc-Cl/
3
pyr; iii)H
O/pyr; iv) RCOCl/pyr; v) piperidine/CH
Cl
.
2
2
2
Later on, it turned out that, in most cases, direct esterification
with the anhydride of choice in MeCN produces the required LG
without any trace of acylation at the exocyclic amino group. Some
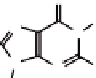
50 different derivatives, having the general formulae
(Chart
4.1) have been prepared so far in our laboratory, mainly in this way.
Besides, we are currently investigating lipophilic guanine derivatives
of type
1a
,
b
,
c
2
, which are prepared mainly via Mitsunobu alkylation [8].
O
O
H
N
N
H
N
X
N
X
RO
N
N
NH
2
O
RO
N
N
NH
2
O
O
O
1a
1b
RO
O
O
H
N
N
H
N
X
N
RO
N
N
NH
2
O
N
N
NH
2
R
1c
2
RO
OR
R = CO-Alk, CO-Ar, Alk
X = H, OMe, OBz, SMe, OH
Chart 4.1





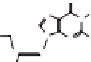





















Search WWH ::

Custom Search