Biology Reference
In-Depth Information
From the very beginning, it was evident that these compounds can
indeed easily self-assemble in organic solvents and that the way they
self-organize mainly depends on the presence of metal ions.
4.2
Self-Assembly in the Presence of Metal Ions
Our experience with guanosine phosphates in water [6], where
G-quartet structures sandwiching alkali metal ions are formed, led
us to try and extract ions from water and transfer them into the
organic phase, in order to obtain G-quartet structures in the organic
solvent [9]. The basic experiment is a very simple one: in a test tube
a yellow water solution of potassium picrate (KPic) and a colorless
chloroform solution of a lipophilic guanosine are shaken for a few
minutes and “the yellow color” migrates from water to chloroform.
As potassium picrate is completely insoluble in chloroform, the
appearance of the color in the organic solution is an evidence of
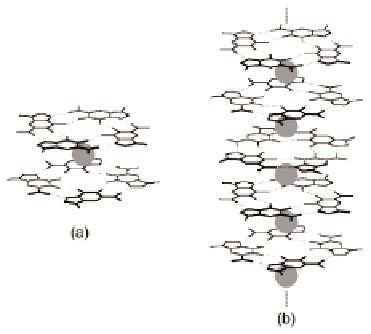
the ionophoric behavior of the LG aggregate. The NMR spectrum
of the LG/K
+
complex was clearly different from that of the LG
molecule before extraction, and the determination of the complex
stoichiometry was straightforward: we had an 8:1 LG/K
+
ratio (the
octamer) when a little potassium picrate was present; with more
potassium picrate, a 4:1 LG/K
+
-polymer)
was formed (Fig. 4.4). NMR and circular dichroism (CD) evidences
indicated that both the octamer and the
structure (the
pseudo
pseudo
-polymer structures
result from G-quartets stacking.
Figure 4.4
Cation (gray sphere) directed supramolecular self-assemblies
of LGs: (a) the octamer and (b) the
pseudo
-polymer.


Search WWH ::

Custom Search