Biology Reference
In-Depth Information
Since those days, thousands of reports have been published on
the biological relevance of G-quartet formation and on the structural
investigation of the arrangements of G-quadruplexes [5].
Depending on the environmental conditions, guanosine
derivatives can either form stacks of the macrocyclic G-quartet
motif or oligomerise through hydrogen bonding to give a variety
of supramolecular networks. This supramolecular setup makes
guanine the most studied homo-nucleobase interaction [1].
Our group has been active in the field of organized systems, and of
liquid crystals in particular, since the early 80s. The research on the
supramolecular behavior of guanine-related compounds started in
our laboratory some 10 years later from the fortuitous observation of
the lyotropic properties exhibited by a guanylic nucleotide in water.
An extensive investigation on several hydrophilic guanosine and
guanine-related derivatives allowed us to demonstrate that these
lyomesophases were originated through a stepwise supramolecular
organization of the guanine bases, where the G-quartet array played
the role of intermediate building block: it is the piling up of G-quartets
that produces the anisotropic rods responsible for the existence of
the mesophases (Fig. 4.3) [6].
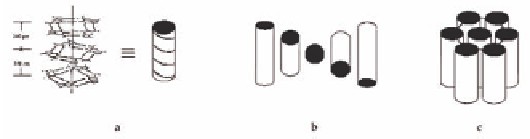
Figure 4.3
(a) Schematic representation of G-quartet stacking, and
the symmetry of the (b) cholesteric and (c) hexagonal
lyomesophases.
Until the mid-1990s it was believed that G-quartet assemblies only
formed in water, and Guschlbauer, a pioneer of the G-quadruplexes
studies, wrote in his 1990 review article [7]: “
water appears to be an
indispensable solvent for the autoassociation of guanosine [...] organic
solvents give rise to poorly organised aggregates.
” Nonetheless, the
nucleobase strong attitude for self-assembly led us to the idea of
preparing LGs for studying the supramolecular behavior of this
nucleobase in the absence of competition for hydrogen bond by
water.


Search WWH ::

Custom Search