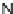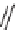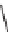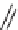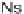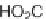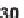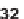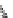Biomedical Engineering Reference
In-Depth Information
SCHEME 3.8
Use of intra- and intermolecular Diels-Alder cycloaddition reactions in com-
bination with metathesis cascade chemistry to produce polycyclic compounds.
First, a dienophile was attached to the substrate followed by an intramolecular
Diels-Alder cycloaddition (
30
). Second, intermolecular Diels-Alder reactions took
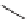
place with different dienophiles (
31
). Third, intramolecular Diels-Alder reactions
occurred between the diene and dienophile, which were both formed in cascade
reactions (
32
).
The Diels-Alder cycloaddition reaction is often used as the last pairing step
in build/couple/pair pathways. Kumagai et al. reported several examples where
Diels-Alder cycloadditions followed enyne ring-closing metathesis reactions. Diene
33
reacted with 4-methyl-1,2,4-triazolin-3,5-dione (MTAD) to afford the tricyclic
compound
34
as a single diastereomer (Scheme 3.9) [22]. The diastereoselectivity
originated from the dienophile approaching the less-hindered side of the pyrroline
ring
33
.
The second build/couple/pair pathway was generated by coupling three simple
monomers, each with electrophilic and nucleophilic functionalities (Scheme 3.10)
[23]. The pair phase, which focused on joining the nonpolar alkene and alkyne
groups using Ru-catalyzed metathesis reactions, generated four final dienes (e.g.,
36
)
that underwent a Diels-Alder reaction with MTAD (e.g.,
37
).