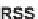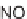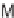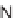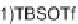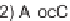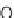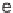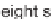Biomedical Engineering Reference
In-Depth Information
SCHEME 17.2
Pair using S
N
Ar.
-amino acid derivative
RR-3
was obtained in three steps from a syn-selective Evan's
asymmetric aldol reaction [22] between
1
and the protected amino acetaldehyde
2
.
The corresponding enantiomers (
SR-3
and
SS-3
, not shown) were also synthesized
starting with the chiral antipodes of
1
and
4
. In the couple phase,
RS-3
was coupled
with the protected alaninol
R-5
and reduced to provide the linear amine
RSS-6
.
All eight stereoisomers of
6
were accessed by a similar sequence of steps, and a
robust synthetic pathway was established to access more than 100 g of each stereoiso-
mer of
6
. The linear template
6
has two amino groups and two hydroxy groups that are
orthogonally protected for a range of chemical modifications leading to a number of
different DOS pathways. More than a dozen cyclic scaffolds have been accessed from
6
, and a representative library synthesis using an S
N
Ar pairing reaction is described
in Schemes 17.2 and 17.3.
The linear amines
6
were acylated with 2-fluoro-3-nitrobenzoyl chloride, and the
resulting amide was subjected to a S
N
Ar cycloetherification on treatment with CsF
[23]. All four diastereomers underwent this cyclization smoothly, and hence all eight
cyclic scaffolds
7
were obtained in high yields. The nitro group was reduced with
Pd/C and the resulting aniline was protected with FmocCl. The Boc group on the
secondary amine was then replaced with an Alloc group to be compatible with the
subsequent solid-phase diversifications. Finally, the primary alcohol was unmasked