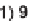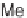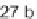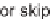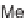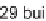Biomedical Engineering Reference
In-Depth Information
SCHEME 17.3
Library production using lanterns.
with the deprotection of the PMB group with DDQ. Using this seven-step sequence,
all eight stereoisomers of
9
were obtained on a scale of 10 to 15 g.
Having assembled all eight stereoisomers of the cyclic core
9
, solid-phase diver-
sifications of both the aniline and secondary amine were undertaken with SynPhase
Lanterns [24] using a split-pool [25] approach. The solid-phase lanterns
10
carrying
the silicon linker were first activated with triflic acid, and then the core
9
was added.
The loading levels for the immobilization step were approximately 15 to 18
mol
per lantern. Washing the lanterns with 20% piperidine in DMF removed the Fmoc-
protecting group. The lanterns were split and the free aniline was functionalized
with 27 building blocks, including sulfonyl chlorides, isocyanates, acid chlorides, or
formaldehyde. Some lanterns also skipped functionalization at this step. The lanterns
were pooled and subjected to alloc deprotection using Pd(PPh
3
)
4
and barbaturic acid.
The lanterns were split again and the secondary amine was functionalized with 29
building blocks, including sulfonyl chlorides, isocyanates, or aldehydes. The lanterns
that skipped the first diversification event also skipped the second diversification
step. Finally, the compound was released from the lantern with HF/pyridine. Using
this split-pool approach, a total of 6448 compounds were obtained from the eight
stereoisomeric cores
9
.
A collection of nearly 20,000 DOS compounds, including the S
N
Ar library
described above, was screened in the phenotypic assay to identify small molecules
that
-cell apoptosis. In parallel, the MLSMR
collection, which contained over 300,000 compounds, was also screened in the same
can
prevent
cytokine-induced