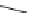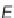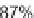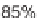Biomedical Engineering Reference
In-Depth Information
SCHEME 9.13
Exploitation of a common intermediate in a branching pathway:
87
,
10 mol% Pd(PPh
3
)
2
(OAc)
2
, benzene, 80
◦
C;
89
,Co
2
(CO)
8
,Et
3
NO-NH
4
Cl, benzene, rt;
90
,
10 mol% CpRu(MeCN)
3
PF
6
, acetone;
88
, 10 mol% Hoveyda-Grubbs's second-generation
catalyst, CH
2
Cl
2
.
compounds
101
to
103
each contain additional functional groups: an alkyne, two
methoxycarbonyl groups, and in the case of
103
, an alkene. Reduction of the nitro
group in
101
triggered cyclization to yield the lactam
105
. Alternatively, conversion
of the nitro group in
102
into a nitrile oxide resulted in a subsequent dipolar cycload-
dition to give the isoxazole
106
. In contrast, ene-yne metathesis of
103
yielded a
1,3-diene substrate for a Diels-Alder reaction (
107
). The reactions of the nitro
compound
104
illustrate how different cyclization reactions may be exploited to yield
alternative scaffolds: metathesis and Diels-Alder reaction to yield
108
, Au-catalyzed
cycloisomerization and lactamization to yield
109
, and Pauson-Khand reaction to
yield the remarkable bridged cyclopentenone
110
.
Barjau et al. have developed a branching pathway based on the versatile chem-
istry of the polycycle
111
, prepared by electrooxidation of 2,4-dimethylphenol
(Scheme 9.16) [32]. A wide range of reactions, several of which are rather remarkable,
were used to prepare skeletally diverse polycycles (e.g.,
112 to 121
). The reactions
included Lewis acid-mediated substitution (
→
→
112
); stereochemically complemen-
tary Wagner-Meerwein rearrangements (
→
113
or
114
), reduction (
→
120
), and
trapping of the open form of the hemiacetal (
121
). In addition, other products,
such as
115
to
119
, were formed by more complex sequences of reactions. Although
a wide range of interesting scaffolds was prepared, the approach does not allow
independent variation of the substituents.
Cui et al. have developed a strategy for the preparation of a shape-diverse library
of compounds with excellent physicochemical properties (Scheme 9.17) [33]. The
→