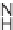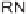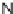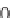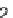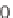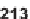Biomedical Engineering Reference
In-Depth Information
SCHEME 7.28
Conversion of silylethers precursors to tetrahydropyrrolo- and
tetrahydropyrido-isoquinolinones and tetrahydroindolizino-indolones. Reagents and condi-
tions: (i) TBAF, AcOH, THF; (ii) Dess-Martin periodinane, DCM; (iii) 10% TFA (aq); (iv)
50% TFA/DCM; (v) 0.1 M NaOH (aq.).
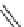
phenylalanine (R
=
H), (3,4-dimethoxyphenyl)alanine (R
=
OMe), and tryptophan
to form precursors
213
(R
H, OMe), and
214
suitable for the following folding
process (Scheme 7.28). The length of a side chain, as well as the amino acid residue,
preencoded the character of the final products.
Deprotection of hydroxyl groups was followed by Dess-Martin periodinane-
mediated oxidation. However, final intramolecular
N
-acyl iminium Pictet-Spengler
reaction only led to products
215
(
n
=
=
0,1) and
216
(
n
=
0); formation of (7
+
6)-fused ring systems (for compounds
215
and
216
) and (6
+
6)-fused ring system
(for compound
216
) failed.
Other examples of the use of a folding process to approach diversity scaffolds
reported by the same authors [53] are discussed in Section 7.5. The folding process
is synonymous for the pair phase, the last step of the B/C/P approach (Scheme 7.39).
An example of a folding process based on regioselectivity has also been described
[57]. Heating of hydrazide precursors
217
and
218
in basic conditions at 80
◦
C
resulted in the formation of either 3-aminohydantoins
219
or 1,2,4-triazine-3,6-diones
220
, depending on the substitution of two nitrogen atoms of the hydrazide moiety
(Scheme 7.29). Resins
217
afforded 3-aminohydantoins
219
. In contrast, when the
hydrogen atom was attached to terminal nitrogen, 1,2,4-triazine-3,6-diones
220
were
obtained instead.