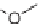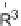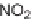Biomedical Engineering Reference
In-Depth Information
SCHEME 7.29
Conversion of hydrazide precursors to 3-aminohydantoins and 1,2,4-
triazine-3,6-diones. Reagents and conditions: (i) DIEA, DMF, 80
◦
C.
7.3 STEREOCHEMICAL DIVERSITY
An intriguing approach to chemical diversity is the preparation of compounds dif-
fering in stereochemistry. These molecules have different three-dimensional spatial
arrangements but identical two-dimensional structures. The most illustrious example
of stereochemical diversity is a combinatorial synthesis of peptides using both L- and
D-amino acids, applied successfully long before the term DOS was introduced [58].
In an analogous manner, using amino acid-based building blocks, stereochemical
diverse spiro-ladder tetramers were synthesized [59]. All of the tetramers were a per-
mutation of four
pro4
monomers (Scheme 7.30). To synthesize a particular oligomer,
monomers
225
were coupled in appropriate sequence to Rink amide resin
221
. Final
tetramers
222
to
224
were cleaved from the resin with 8% TFMSA in TFA, precipi-
tated, and dissolved in 20% piperidine in NMP to trigger closure of the second amide
bond between adjacent residues.
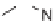
Another example focusing exclusively on stereochemical diversity is the synthesis
of pyrrolo[3,4-
c
]pyrrole and pyrrolidine derivatives from Merrifield resin-supported
-Silylimines
226
were treated with dipolarophiles to yield two
heterocyclic cores in two and four diastereomeric forms, respectively (Scheme 7.31).
First, the Ph derivative (R
-silylimines [60,61].
Ph) was examined. When the reaction proceeded
at 180
◦
C for 6 h, the two diastereoisomers
227
and
228
were obtained in the ratio
36 : 64 (the stereoselectivity was rather low under those conditions). As expected,
when the reaction was carried out at 40
◦
C for 48 h, the ratio changed in favor of
diastereoisomer
227
(the ratio was 93 : 7). The cycloaddition was then carried out
with other para-substituted phenyl derivatives. Even though the ratio was influenced
by the substitution, more than 74% of cycloadduct
227
was formed in all cases.
=