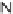Biomedical Engineering Reference
In-Depth Information
cyclopentapyrrolidinone scaffolds as single diastereomers (
102
). The Rh-catalyzed
butadiene [4
2] cycloaddition reaction developed by Evans [63], after oxidation
to the corresponding
t
-butylsulfonamides, afforded [5,8]-bicyclic cyclooctapyrroli-
dine scaffolds (
103
) diastereoselectivily, but with moderate yield. Several [2
+
2
+
2]
cyclotrimerization were also identified for the diynes. Reaction of diyne
101
with
propargyl alcohol in the presence of Grubbs's first-generation catalyst provided
[5,6]-bicyclic isoindoline scaffolds (
104
) with complete regioselectivity [64]. Treat-
ment of the diyne with benzyl isocyanate and Yamamoto's Ru(II) catalyst afforded
[5,6]-bicyclic pyrrolopyridone scaffolds (
105
) [65]. The diyne also cyclotrimerized
with ethyl cyanoformate efficiently using Tanaka's Rh(I) catalyst [66], providing
[5,6]-bicyclic pyrrolopyridine scaffolds (
106
) regioselectively. Finally, [5,7]-bicyclic
cycloheptapyrrolidine scaffolds (
107
) were synthesized through [3
+
2
+
2] Ni(0)-
catalyzed cyclotrimerization [67] of the diyne with ethyl cyclopropylideneacetate.
The reaction provided single regioisomers, but inseparable
E
/
Z
mixtures.
In an effort to develop new chemical methodologies for the production of diverse
sultam-based libraries, Samarakoon et al. recently reported a one-pot, complemen-
tary ambiphile-pairing (CAP) reaction to produce 5,2,1-dibenzooxathiazocine-2,2-
dioxides (
108
) in a formal [4
+
2
+
4] cyclization pathway (Scheme 3.36) [68]. In this
CAP strategy,
o
-quinone methides (
o
-QMs) and
o
-fluorobenzensulfonamides were
utilized as ambiphilic synthons.
o
-QMs are powerful intermediates both as Michael
acceptors and as dienes in cycloaddition reactions, but they have never been reported
as ambiphiles in a hetero [4
+
+
+
4]
cyclization reaction occurred in which aza-Michael addition at the
exo
-methylene
o
-
QM carbon was followed by nucleophilic addition to
o
-fluorobenzenesulfonamides
via S
N
Ar reaction.
When the
o
-QM was replaced by an epoxide, a formal [4
4] cyclization. In the pair phase, a formal [4
3] epoxide cascade
reaction occurred with epoxide ring opening followed by either an S
N
Ar or oxa-
Michael cyclization to provide benzothiaoxazepine-1,1
-dioxide and oxathiazepine-
1,1
-dioxide scaffolds, respectively (
109
,
110
) (Scheme 3.37) [69]. In both cases, the
reaction tolerated several epoxides and sulfonamides, demonstrating the versatility
and extent of this pathway.
+
SCHEME 3.36
Formal [4
+
4] cyclization of
o
-quinone methides and
o
-fluorobenzen-
sulfonamides.