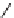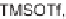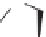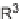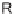Biomedical Engineering Reference
In-Depth Information
SCHEME 3.37
Formal [4
+
3] epoxide cascade followed by either an S
N
Ar or oxa-Michael
cyclization.
2] annulation of sub-
stituted
N
-methylisatin dimethyl ketals and enantioenriched crotyl sylanes [70] to
access spirooxindoles with excellent stereocontrol (Scheme 3.38) [71]. The cis iso-
mer
111
was the main product when lower temperature and a shorter reaction time
were employed. A longer reaction time or more polar solvents favored the trans iso-
mer
112
, suggesting that the cis isomer might epimerize through a spiro-ring-opening
mechanism. Interestingly, the cis product
111
could easily be converted to the trans
isomer
112
in the presence of BF
3
·
Zhang and Panek developed a Lewis acid-catalyzed [5
+
OEt
2
under microwave irradiation. This made it
possible to access all possible stereoisomers for elucidating stereostructure-activity
relationships during screening campaigns.
A proposed mechanism is illustrated in Figure 3.6. Because of the absence of
peri-like interaction, the transition state
113
is favored over the (
E
)-oxonium inter-
mediate
114
, and generates the cis as the main diastereomer under kinetic conditions.
Finally, the complexity of the spirooxindoles can be enhanced by employing different
combinations of functionalized silyl alcohols and functionalized isatines.
Panek's group later extended this work by combining the use of enantioenriched
crotyl sylanes with rhodium(II)-catalyzed asymmetric cyclopropanation to generate
cyclopentene compounds as building blocks for the synthesis of small-molecule
libraries (Scheme 3.39) [72,73]. Cyclopropanation reaction, followed by Lewis
SCHEME 3.38
2] Annulation of substituted
N
-methylisatin dimethyl ketals and enan-
tioenriched crotyl sylanes.
[5
+