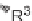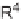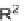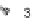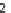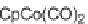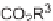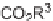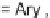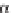Biomedical Engineering Reference
In-Depth Information
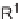
SCHEME 3.33
Cobalt-catalyzed [2
+
2
+
2] cycloaddition reaction of silyl ether-tethered
diynes with nitriles.
of silyl ether-tethered diynes with nitriles (Scheme 3.33) [58]. The tether plays a key
role by controlling the regioselectivity and allowing for transformation of the bicyclic
intermediates into final highly functionalized monocyclic pyridines. CpCo(CO)
2
was
found to be the best catalyst, and the use of THF as a solvent, inducing catalyst
turnover, allowed an irradiation-free reaction. A large variety of nitriles was well
tolerated for this reaction, with the exception of strongly deactivated and pyrazine-
containing nitriles, and nitriles with free hydroxyl and amine groups. For example,
aromatic, heteroaromatic, benzylic, and primary and secondary aliphatic nitriles eas-
ily underwent cycloaddition reaction, giving the desired products. Large substituents
on the silylated alkyne were also well tolerated, and since the distal alkyne did not
need to be terminal, its substituents allowed access to fully substituted pyridines. One
of the desilylated pyridines was discovered to inhibit the neuregulin/Erb4 pathway,
which appeared likely to be involved in breast cancer and schizophrenia [59].
Guo et al. reported an elegant phosphine-promoted [3
3] annulation of aziridines
and allenoates to generate highly substituted tetrahydropyridines
99
(Scheme 3.34)
[60]. The authors started with the idea that the phosphonium enolate zwitterionic
intermediate may interact with the aziridines and give the desired product. PPh
3
was found to be the best phosphine source when
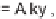
N
-nosylaziridine
97
and diethyl
2-vinylidenesuccinate
98
were used. The reaction proceeded with excellent diastere-
oselctivity (trans/cis 10 : 1). Interestingly, the presence of the
+
β
carbon
α
,
β
, and
atoms of the starting allenoate in the final core, rather than
α
,
β
, and
γ
as with
SCHEME 3.34
Phosphine-promoted [3
+
3] annulation of aziridines and allenoates to gen-
erate highly substituted tetrahydropyridines.