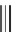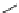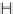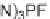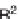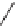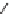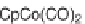Biomedical Engineering Reference
In-Depth Information
SCHEME 3.31
Ruthenium-catalyzed [5
+
2] cycloaddition reaction and Pauson-Khand
reaction to generate azabicyclic compounds.
diene
93
through a cyclopropyl ring-opening/reductive elimination sequence
(Scheme 3.31). The reaction proceeded diastereolectively to afford a single diastere-
omer. A Pauson-Khand reaction, a [2
1] cycloaddition, was also reported. In
the presence of trimethylamine
N
-oxide, the azabicyclo-[3.3.0]
94
was synthesized
with high diastereoselectivity (
+
2
+
10 : 1 dr).
Zhou et al. developed a cobalt-catalyzed intramolecular [2
>
2] cyclization
reaction of dialkynylnitriles to provide tetrahydronaphthyridines scaffolds, which,
compared to fully aromatized naphthyridine, have not received particular attention
in drug discovery (Scheme 3.32) [57]. CpCo(CO)
2
was found to be the best cat-
alyst, and by changing the length of the aminonitriles, the authors were able to
access 5,6,7,8-tetrahydro-1,6-naphthyridines (
n
+
2
+
=
2), 6,7-dihydro-5
H
-pyrrolo[3,4-
b
]pyridines (
n
=
1), and 6,7,8,9-tetrahydro-5
H
-pyrido[2,3-
d
]azepines (
n
=
3).
Tetrahydropyranonaphtyridine (
n
2)
95
was chosen for library production. With
this strategy, several substituents at
C
6 and
C
8 were introduced and the secondary
amine at
N
2 was subsequently used as the diversification point for the formation of
ureas, amides, and sulfonamides.
Gray et al. described a build/couple/pair strategy to synthesize highly substituted
pyridines
96
through a transition metal-mediated [2
=
+
+
2
2] cycloaddition reaction
SCHEME 3.32
Cobalt-catalyzed intramolecular [2
+
2
+
2] cyclization reaction. (Adapted
from [57], with permission; copyright
C
2008 American Chemical Society.)