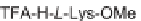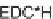Biomedical Engineering Reference
In-Depth Information
SCHEME 3.29
Regioselective 1,3-dipolar cycloaddition reaction to generate 1,4- and 1,5-
disubstituted triazoles.
activates the siloxy alkyne, followed by 1,4-addition and trapping of the ketenium
ion. Interestingly, the resulting siloxy cyclobutenes can be further functionalized
through their ester, nitrile, and ketone functionalities, allowing for the synthesis of
small libraries.
Using intramolecular pairing cyclization reactions of nonpolar-nonpolar and
polar-nonpolar groups strategically placed on highly functionalized amino alco-
hols, Kumagai et al. generated a short modular synthetic pathway to access 15
different scaffolds in only five steps [22]. A ruthenium-catalyzed [5
2] cycloaddi-
tion reaction between the nonpolar alkene and alkyne groups afforded the cyclic
+
SCHEME 3.30
Silver-catalyzed [2
+
2] cycloaddition of siloxy alkynes with esters, nitriles,
and ketones.