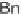Biomedical Engineering Reference
In-Depth Information
FIGURE 3.5
Synthesis of macrocyclic peptidomimetic compounds; triazole and monoke-
topiperazine moieties are introduced as amide bioisosteres.
reaction to afford triazolo-1,5-benzodiazepine-2-ones
91
. This scaffold is present in
compounds that inhibit serine proteases and bind to the benzodiazepine receptor [53].
Inspired by the large number of biologically active molecules containing peptide
motifs, Isidro-Llobet and co-workers designed and developed a DOS strategy for the
generation of macrocyclic peptidomimetic compounds, which are still underrepre-
sented in current small-molecule libraries [54]. A triazole ring and a ketopiperazine
moiety were introduced as bioisosteres of the amide to improve the instability of
the peptides [55]. Besides conferring rigidity, triazoles also mimic either the cis- or
trans-like configuration of the amide bond (Figure 3.5). In a proof-of-concept study,
they reported the synthesis of 12 compounds with four different scaffolds:
cis
-DKPs,
trans
-DKPs, and 1,4- and 1,5-triazoles.
A click-type 1,3-dipolar cycloaddition reaction was used to build the macrocyclic
triazole-based peptidomimetic. Cu(I) and [Cp*RuCl
4
] were used as catalysts to syn-
thesize 1,4- and 1,5-disubstituted triazoles, respectively. The 1,3-dipolar cycload-
dition reactions were fully regioselective, and no epimerization of the stereogenic
center adjacent to the azide occurred (Scheme 3.29).
3.4 MISCELLANEOUS CYCLOADDITIONS
Several other cycloaddition reactions have been used in DOS for their ability to
introduce structural and skeletal diversity. In line with their interest in the reactivity
of siloxy alkynes, Sweis et al. reported the discovery of the first silver-catalyzed
[2
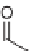
2] cycloaddition of siloxy alkynes with esters, nitriles, and ketones [56]. This
transformation is an efficient and versatile method to access highly functionalized
siloxy cyclobutenes (Scheme 3.30). AgNTf
2
was found to best effect the cycloaddition
of
+
-unsaturated ketones, nitriles, and esters with siloxy alkynes to yield the
corresponding siloxy cyclobutenes
92
. In addition, [4.2.0]bicyclic silyl enol ether
was synthesized when using cyclohexenone. Aryl-substituted siloxy alkynes were
also well tolerated. (
E
)- and (
Z
)-crotonates gave the same trans-substituted siloxy
cyclobutene, suggesting that the reaction proceeds via a stepwise mechanism. Silver
α
,
β