Biomedical Engineering Reference
In-Depth Information
0.4
0.35
0.3
0.25
0.2
Control
Fc-OX40 L
OX86
0.15
0.1
0.05
0
5
7
9 12
Days after tumor implantation
14
16
20
22
FIGURE 19.5
Immunotherapy response with Fc-OX40L and agonist anti-OX40 antibody (OX86)
in COLON 26-bearing BALB/c mice.
co-stimulates CD8
þ
T cells [76]. Profound CD8
þ
T cell
cancer [83]. This ADEPT strategy is a two-step approach
which seeks to generate a potent cytotoxic agent selectively
at a tumor site [31]. In the first step, a tumor selective
antibody linked to an enzyme (fusion) is administered and
allowed to localize at the tumor site. This is followed, in the
second step, by the administration of a relatively nontoxic
prodrug that will be enzymatically converted to active drug
only at the tumor site (optimum at low pH) to minimize the
usual nonspecific toxicity. Potentially, one of the major
advantages of ADEPT is the ability to deliver, to the tumor,
a highly potent cytotoxic drug in concentrations that cannot
be achieved by conventional chemotherapy. In principle, to
maximize the benefit of ADEPT therapy it is beneficial to
(a) have high tumor to normal tissue ratio of the conjugate,
(b) ensure the prodrug to drug conversion is maximal at the
tumor site, and (c) generate a potent drug that will act locally
and not diffuse into normal tissues.
To test this hypothesis, fusion proteins consisting of
single chain Fv (scFv), Fab, or F(ab')
2
fragments of
chTNT-3 and the human
b
G enzyme were constructed for
ADEPT [84]. Each of these reagents was tested to assess
specificity and avidity of antigen binding as compared to the
parental antibody. In addition, studies were performed to
demonstrate enzymatic function of the fusion proteins and
retention of catalytic activity in circulating blood, specific
tissues, and tumor after in vivo administration. The results of
these experiments show that all three constructs retained
their antigen binding capability and demonstrated active
enzymatic function against substrate in vitro. Pharmaco-
kinetic and biodistribution studies of radiolabeled fusion
proteins in MAD109 murine lung carcinoma tumor model
transplanted into BALB/c mice confirmed specific localiza-
tion of the fusion proteins and rapid clearance from blood
and normal tissues over time. For example, tumor uptake
(% injected dose per gram) of the fusion proteins at 1 day
postinjection varied from 2.35% for scFv/
b
G to 2.52% for
Fab/
b
G, and to 2.91% for F(ab
0
)
2
/
b
G. Despite this rather
low uptake,
clonal expansion and a massive burst of CD8
T cell
effector function were induced by dual co-stimulation of
OX40 and CD137 [77]. This synergistic response of the
specific CD8
þ
þ
T cells has been shown to last for several
weeks, and is sufficient to treat established tumors even
under immunocompromised conditions.
Interestingly, OX40 is also found to be expressed on both
na
T
reg
cells [79]. OX40
signals may abrogate T
reg
-mediated suppression when they
are delivered directly to antigen-engaged na
ıve and activated CD4
þ
CD25
þ
€
ıve T cells [80].
Using the agonist antibody, OX86, OX40 signaling on T
reg
cells inhibited their capacity to suppress, and restored
effector T cell proliferation and cytokine production [81].
It has been confirmed that OX40 abrogation of T
reg
cells
occurs in vivo using a graft-versus-host disease model [81].
Since the C-terminus of OX40L is extracellular and
essential for its bioactivity, the N-terminus of the extrac-
ellular OX40L gene was fused to the C-terminus of the
immunoglobulin HC gene [82]. A dosing study was
performed on COLON 26-bearing BALB/c mice with Fc-
OX40L and the anti-OX40 agonist antibody OX86 (Figure
19.5). Treatment groups receiving high doses of Fc-OX40L
showed 80-90% tumor reduction, whereas the OX86-treated
mice achieved less tumor reduction. This difference poten-
tially indicates that at this particular dosage, the Fc-OX40L
is more effective. Identical treatment studies performed with
the soluble fusion protein showed similar regression of
RENCA tumors. All treated groups eventually became
tumor free and were free of signs of toxicity throughout
the 120-day observation period.
€
19.3.4 Enzyme Fusion Proteins
Mammalian enzymes, such as carboxypeptidase A and
b
-glucuronidase (
b
G) that are unlikely to be found in
high basal concentrations in the body, have been also
used resulting in ADEPT technology for the treatment of
the rapid clearance of the fusion proteins

































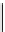















Search WWH ::

Custom Search