Biomedical Engineering Reference
In-Depth Information
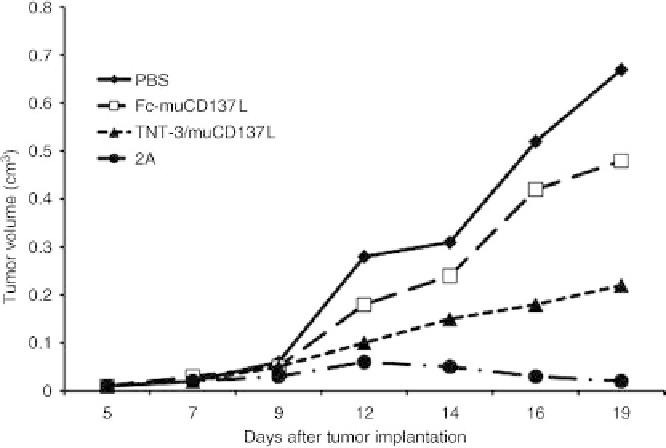
FIGURE 19.4
Dose response of mTNT-3/CD137L, Fc-CD137L, and agonist CD137 antibody 2A
in COLON 26-bearing BALB/c mice.
T
reg
-induced suppression. One such cell surface marker is a
member of TNFSF designated GITR, which is constitutively
expressed on CD4
that received the maximum dose (90
m
g) of Fc-GITRL
protein displayed the longest survival out of all of the
treatment groups. In addition, the dosing study of
GITRL-Fc demonstrated an 87% reduction in tumor volume
at a dose of 11
m
g compared to the control LFA-Fc treatment
group. Therefore, these data show that treatment using as
little as 11
m
g of either Fc-GITRL or GITRL-Fc can activate
an effective anti-tumor response in vivo.
þ
CD25
þ
(T
reg
) cells and is up-regulated
in CD4
cells [69]. GITR has a high homology
in the cytoplasmic region with other TNFSF members such
as CD137, OX40, and CD40L. The ligand for GITR
(GITRL) is a type II transmembrane protein that belongs
to TNFSF and is normally expressed on APCs such as
macrophages, dendritic cells, and B cells. More importantly,
GITR activation by GITRL leads to the proliferation of
CD4
þ
and CD8
þ
19.3.3.4 OX40L Fusion Proteins
OX40 (CD134), a
membrane-associated glycoprotein, is transiently expressed
on the surface of T cells after TCR ligation [71]. Its natural
ligand, OX40L (TNFSF4, CD134L), is found primarily on
APCs such as activated B cells, activated endothelial cells,
dendritic cells, and macrophages [72,73]. The OX40L deliv-
ers a potent co-stimulatory signal to OX40
CD25
and CD8
effector T cells and a reduction
of tumor volume in vivo. Also, an interesting characteristic
of GITR activation on T
reg
cells results in their inability to
suppress responding CD4
þ
þ
CD25
T cells [66]. Therefore,
GITR engagement by GITRL results in the inhibition of T
reg
cells and the activation of responding T cells. This dual
function of GITR activation may be unique for co-stimula-
tory interactions and could be responsible for its highly
potent anti-tumor activity in vivo.
Both C- and N-terminal Fc fusion proteins containing the
extracellular domain of murine GITRL were also con-
structed [70]. In order to determine the therapeutic efficacy
of the GITRL fusion proteins, immunotherapy studies were
performed with tumor-bearing BALB/c mice using a
COLON 26 tumor model. The activity of Fc-GITRL
(C-terminal) and GITRL-Fc (N-terminal) was first examined
at different doses in order to analyze its potency in vivo. For
comparison, Fc fusion proteins consisting of the lymphocyte
function-associated antigen (LFA) were used in several
aspects of this work. The group that received 11
m
gof
Fc-GITRL fusion protein or the maximum dosage of
90
m
g both resulted in a 96% tumor reduction. The group
þ
T cells leading
to optimal T cell function. Studies of the primary T cell
responses revealed that OX40L is a potent co-stimulatory
molecule for sustaining CD4
þ
T cell response [74]. OX40
signaling strikingly prolongs T cell division initially induced
by CD28, and enhances the survival of CD4
þ
cells during
the initial response by promoting Bcl-XL and Bcl-2 expres-
sion. OX40
þ
T cells fail to maintain high levels of Bcl-
XL and Bcl-2 4-8 h after activation, and undergo apoptosis
[75]. In vivo, OX40 signaling would augment tumor-specific
priming through stimulating and expanding the natural
repertoire of the host's tumor-specific CD4
/
T cells [73].
Therefore, it is believed that OX40-OX40L interactions are
crucial for the survival of effector T cells and the generation
of memory T cells. Although the effects of OX40 co-
stimulation on CD4
þ
T cells were initially studied,
more recent work has shown that OX40 signaling also
þ
Search WWH ::

Custom Search