Biomedical Engineering Reference
In-Depth Information
Control (no treatment)
Control (Fab/Glucuronidase)
Doxorubicin: 4 mg/kg
Prodrug only 200 mg/kg
Fab/Glucuronidase: 10 mg/kg + prodrug 100 mg/kg
Fab/Glucuronidase: 10 mg/kg + prodrug 200 mg/kg
Fusion protein treatment
6
Prodrug treatment
5
4
3
2
1
0
8
10
12
14
16
18
20
22
24
26
Days
FIGURE 19.6
Treatment of MAD109 in BALB/c mice using combination of the Fab/human
b
-glucuronidase fusion protein and a prodrug of doxorubicin.
produced higher tumor/normal organ ratios (
1) for many
normal tissues, illustrating the specificity of tumor targeting
with the fusion proteins and their rapid elimination from the
blood and normal organs. Finally, one of the constructs
(chTNT-3 Fab/
b
G was used in a pilot treatment study
with a glucuronide prodrug of doxorubicin to demonstrate
the anti-tumor activity of ADEPT using the chemoresistant
MAD109-bearing BALB/c mice. As shown in Figure 19.6,
fusion protein pretreatment was very effective in slowing the
rate of tumor growth. Although doxorubicin-treated mice
showed a slow rate of growth, the prodrug-treated groups
demonstrated more profound effects and had outstanding
anti-tumor responses compared to control groups. Impor-
tantly, no toxicity or adverse effects were seen. In addition,
the group receiving the combination fusion protein and
higher dose of prodrug (200 mg/kg) had a higher survival
rate (62%) compared to the group treated with doxorubicin
alone (43%). These studies show that the use mAbs to target
the enzyme to the tumor is a significant advance in ADEPT
and that further studies are warranted to test this novel
therapeutic approach in the treatment of solid tumors.
of the extrinsic pathway of the blood coagulation cascade
and normally released from damaged tissues [85]. By
substituting the attachment site with a tumor delivery agent,
this potent thrombogenic protein in its tTF form can be
targeted to the tumor where it can initiate clotting, thereby
occluding the tumor's blood supply and causing rapid tumor
destruction. Several advantages of this approach over con-
ventional antitumor therapies have been suggested [27]
including (a) the target molecules are directly accessible
to antigen, permitting rapid localization of a high percentage
of the injected dose; (b) cellular degeneration caused by the
occlusion of tumor vessels is microregional, amplifying the
effects of therapy; (c) microvascular endothelial cells are a
normal, genetically stable cell population, so target antigens
remain relatively the same regardless of selective pressures
exerted by cytotoxic therapies; and (d) the same target drug
can be used for a variety of solid tumors because tumor
vessels share common morphological, immunological, and
biochemical properties.
To test the therapeutic potential of this vascular targeting
approach, three fusion proteins, chTNT-3/tTF, chTV-1/tTF,
and RGD/tTF, which target DNA exposed in degenerative
areas of tumors, fibronectin on the tumor vascular basement
membrane, and
a
n
b
3 on the luminal side of tumor vessels,
respectively, were developed and tested for their anti-tumor
effects [28]. Antigen binding and clotting assays demon-
strated that each of the fusion proteins retained their antigen
binding and thrombogenic activities. In vivo studies in
COLON 26-bearing mice revealed that all three reagents
induced histological evidence of microregional thrombosis
>
19.3.5 Vascular Targeting Fusion Proteins
The primary objective of this approach is to explore the use
of mAbs to deliver potent cytotoxic agents capable of
inducing sustained, effective therapy that selectively block
the blood flow to tumors by targeting different antigens. A
potential mediator of this event is the vascular cell mem-
brane receptor protein tissue factor (TF) that is the initiator
































































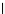







































































Search WWH ::

Custom Search