Biomedical Engineering Reference
In-Depth Information
CH
2
OH
CH
2
OH
H
H
H
H
O
O
C+
O
H
C
OH
O
O
OH
OH
*
*
*
*
H
*
H
OH
O
N
NH
CH
2
OH
n
n
H
H
HO
+n
CH
OH
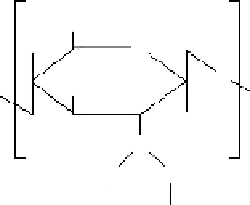
Figure 1.14
Reaction of chitosan and formaldehyde hydrate.
The Schiff base of formaldehyde and chitosan can be reduced by NaBH
3
CN to form
N
-methyl chitosan [74]. The method comprises the following steps [75]: suspending 20 g
of chitosan powder with 2 L of water, adding 20 mL of glacial acetic acid to the suspen-
sion, stirring to dissolve glacial acetic acid, adding formaldehyde of the calculated
amount in two batches, stirring for 30 min, adjusting the pH to 4.5 by using NaOH solu-
tion, dissolving 5 g of NaBH
4
in 50 mL of water, adding the solution to the reactant in 1 h
during stirring, reacting for 1 h until the pH is 5.5, adjusting the pH to 10 by using alkali
until
N
-methyl chitosan deposits, filtering, washing deposits with water to neutral, and
extracting them with ethanol and ether via a Soxhlet extractor to remove the remaining
formaldehyde and inorganic compounds.
A similar method can be used to prepare
N
-dimethyl chitosan: adding 50 mL of glacial
acetic acid to 2 L of water, adding 50 g of 100-200 mesh chitosan to the solution, stirring the
mixture to dissolve solid and form a solution of pH 3.2, adding 500 mL of 35% formalde-
hyde solution, stirring strongly to immediately form
N
-methylene chitosan gel, keeping it
still for 12 h, adding 13 g of NaBH
4
in 8 h, reacting at 15-20°C until the pH is 4.0, which
shows that the reaction is complete, adjusting the pH to 9.0 by using NaOH solution, wash-
ing the gel with water, and using acetone for depositing, filtering, and extracting deposits
by using ether in a Soxhlet extractor to obtain a white powder that is insoluble in acetic
acid or hydrochloric acid.
A small amount of formaldehyde cross-links chitosan, increasing the viscosity and even
changing the solution into a gel. The specific steps are as follows: swelling chitosan by 95%
ethanol for 2 h, adding excess salicylaldehyde to the mixture, stirring in reflux for 8 h,
filtering to harvest the yellow product, extracting it by using ethanol in a Soxhlet extractor
for 24 h to get salicylaldehyde chitosan Schiff base, and reducing it by NaBH
4
in methanol
CH
2
OH
O
O
OH
*
*
N
n
H
2
C
CH
2
OH
OH
Figure 1.15
N
-dihydroxylmethyl derivative of chitosan.
Search WWH ::

Custom Search